Advances in Animal and Veterinary Sciences
Research Article
Evaluation the Antibacterial Properties of Different Extracts of Cinnamomum zeylanicum Barks
Ali H. Saliem*, Ahmed N. Abedsalih
College of Veterinary Medicine, University of Baghdad, Iraq.
Abstract | Antibacterial activities of different concentrations of methanolic and chloroform Cinnamomum zeylanicum bark extract were studied against a number of pathogenic isolates included Streptococcus pyogenes, Enterococcus faecalis and Escherichia coli and compared with ampicillin using agar well diffusion method. Two types of solvents were used for extraction, methanol and chloroform, with different concentrations (100µg/µl,150µg/µl, and 200µg/µl) for each extract were used. The results revealed that the pattern of inhibition varied with the solvent used for extraction and concentration. Extract that prepared in methanol provided more antibacterial activity as compared to chloroform extract against all tested bacteria at all concentrations, where the Streptococcus pyogenes was more susceptible than and Enterococcus faecalis and Escherichia coli respectively to both extracts. Results of the present study sign the interesting assurance of designing an active antibacterial agent from Cinnamomum zeylanicum bark.
Keywords | Antibacterial, Properties, Cinnamomum zeylanicum, Barks, Extracts.
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | June 09, 2018; Accepted | August 10, 2018; Published | August 27, 2018
*Correspondence | Ali H Saleim, College of Veterinary Medicine, University of Baghdad, Iraq; Email: alisaliem977@gmail.com
Citation | Saliem AH, AbedSalih AN (2018). Evaluation the antibacterial properties of different extracts of cinnamomum zeylanicum barks. Adv. Anim. Vet. Sci. 6(9): 380-383.
DOI | http://dx.doi.org/10.17582/journal.aavs/2018/6.9.380.383
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2018 Saliem and Abedsalih. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Cinnamomum zeylanicum is a small, tropical, evergreen tree most noted for its bark, which provides the world with the commonly known spice, cinnamon. Cinnamomum zeylanicum is the scientific name, which refers to the specific species of tree that cinnamon is harvested from (Brierley, 1994; Bown, 1995). In folk medicine was once used as a cure for colds. It has also been used to treat diarrhea and other problems of the digestive system. C. zeylanicumbark is high in antioxidant activity (Shan et al., 2005; Priyanga et al., 2013). The essential oil of cinnamon also has antimicrobial properties, which can aid in the preservation of certain foods (Lopez et al., 2005; Vaibhavi et al., 2010). C. zeylanicum bark has been reported to have remarkable pharmacological effects in the treatment of type II diabetes and insulin resistance (Verspohl et al., 2005). C. zeylanicum bark is rich in terpenoids, including linalool, eugenol, and methyl chavicol, and in chemicals, including resinous compounds, cinnamaldehyde, ethyl cinnamate and caryophyllene, Cinnamic acid, L-borneol, L-bornyl acetate, E-nerolidol, and cinnamyl acetate which contribute to its distinct aroma (Jayaprakasha et al., 2002). In addition, some protein is also present in the bark. These substances are believed to play an important role in the antibacterial activity (Tung et al., 2008). This work was aimed to evaluate the antimicrobial properties of C. zeylanicum bark extracts against Streptococcus pyogenes , Enterococcus faecalis, and Escherichia coli.
Methods
Plant Materials
Cinnamon zeylanicum barks that were collected from the local market in Baghdad were dried naturally in room temperature at shade for a week for complete moisture removal. The barks were cut into small pieces, crushed into a fine powder by an electrical grinder then sieved using a 20-mesh sieve to get uniformed size range. The final sieved powder was used for all further studies (Gauthami et al., 2015).
Preparation of Cinnamon zeylanicum Barks Extract
Two forms of extraction with methanol 70% and chloroform were made. The extraction done by putting 2.5gm of prepared powder of Cinnamon zeylanicum in 100 ml of each solvent, the mixture stirred and filtered with gauze then by filter paper and freed from solvent by incubation. Crude concentrated extract was stored at 4°C until used for further testing (Gauthami et al., 2015).
Bacterial Isolates Activation and Maintenance
All the tested pathogenic bacteria were obtained from the College of Veterinary Medicine/ University of Baghdad. Bacterial cultures were activated in screw-capped tubes containing 10 ml of brain heart infusion agar slants and incubated for 24 hours at 37oC then the isolates identified by the routine tests. For maintenance of isolates, the media were stored at 4oC, and were subcultured once every two-weeks for further investigation (Tomar et al., 2010).
Antibacterial Assay
Preparation of Standard Bacterial Suspension: The mean numbers of the viabletested bacteria (Streptococcus pyogenes, Enterococcus faecalisand Escherichia coli) of the stock suspensions was measured by the average of the standard McFarland solution No.0.5 by taking 1 ml from over-night cultures [brain heart infusion broth (BH)] of each bacterial suspension mixing with 9 ml of peptone water, then taking 1 ml of this suspension and making serial ten-fold dilution (Quinn et al., 2004).
Preparation the Concentration of Antibacterial: Stock solution of ampicillin was prepared by mixing 0.1 gram with 10 ml of distilled water (10mg/ml), then concentrations of (0.2µg/µl) were prepared by mixing known volume from the stock solution with distilled water.
Preparation of Different Concentrations of Cinnamomum zeylanicum Extract: Stock solution of each extract was prepared by mixing 0.1 gram with 10 ml of distilled water (10mg/ml), then concentrations of (100µg/µl, 150µg/µl and 200µg/µl) were prepared by mixing known volume from the stock solution with distilled water. These concentrations of extracts and antibacterial were used in sensitivity test to determine the tested bacteria sensitivity to extracts and antibacterial agent.
Sensitivity Test
Sensitivity test of the Cinnamomum zeylanicum extracts compared with an antibacterial agent (ampicillin). The method of agar well diffusion was adopted according to Kavanagh (Kavanagh, 1972; Perez et al., 1990). 0.5ml of standardized stock suspensions (1.5 ×108 CFU/ml) of each tested bacteria (Streptococcus pyogenes, Enterococcus faecalis, and Escherichia coli ) was mixed to 500 ml of sterile Mueller Hinton agar at 45oC. Fifteen milliliters of the inoculated Mueller Hinton agars were poured into the sterile Petri dishes of each. The agars were left to set for 10 minutes to permit solidifying the agar then making wells in these plates in a diameter 6 mm after that the wells were filled with 55 microliters of each concentration of each extract (100µg/µl, 150µg/µl,and 200µg/µl) and ampicillin 0.2µg/ µl. The plates then incubated at 37oC in the upright position for 24 hours. Three replicates were done for each concentration of both extracts and the activity was dictated by measuring the distance across (diameter) of inhibition zone around every well by millimeter against tested bacteria.
Statistical Analysis
Statistical analysis of data was performed using SAS (Statistical Analysis System - version 9.1). One-way ANOVA and Least significant differences (LSD) post hoc test was performed to assess significant difference among means.. P < 0.05 was considered statistically significant.
Results and Discussion
Different concentrations of methanol and chloroform extracts (100µg/µl,150µg/µl, and 200µg/µl) and ampicillin (0.2µg/µl) were used in agar well diffusion assay and caused different degrees of inhibition against tested bacteria with diameter of zone inhibition ranged from 6.20±0.13mm–9.60±0.03mm for Streptococcus pyogenes, 4.40±0.18mm–8.00±0.06mm for Enterococcus faecalis and 0.00±0.00mm–6.20±0.03mm for Escherichia coli (Table 1),
(Figure 1). The size of inhibition zones was proportionally increased with increasing of concentration of the agents.
The results showed that the standard antibacterial (ampicillin) more potent against tested bacteria with significant difference (P<0.05) as compared with all used concentrations of both methanol and chloroform extract. Also the antibacterial activity of methanol extract was more effective than chloroform extract against all tested bacteria in all concentrations with significant difference (P<0.05) except between 200µg/µl of chloroform and 150µg/µl of methanol against Streptococcus pyogenes and between 200µg/µl of chloroform and 100µg/µl of methanol against Escherichia coli where the differences were not significant. The results also revealed there was a significant difference (P<0.05) in sensitivity of the tested bacteria for both extracts, where the Streptococcus pyogenes was more sensitive than Enterococcus faecalis and Escherichia coli respectively. The results of this study were agree with several data obtained by (Usha et al., 2012; Hassan et al., 2014; Tomar, and Shrivastava, 2015; Abbaszadegan et al., 2016; Alsalim et al., 2016) who reported that the Cinnamomum zelanicum has antibacterial activity against gram-positive and gram-negative bacteria including Streptococcus pyogenes, Enterococcus faecalis, and Escherichia coli.
Table 1: Antibacterial activity of Cinnamomum zeylanicum bark extracts (methanol and chloroform) and reference antibiotic (ampicillin) in different concentrations against Streptococcus pyogenes, Enterococcus faecalis, and Escherichia coli.
| Extract | Concentration µg/µl | Zone of inhibition (mm) | ||
| Strep. pyogenes | Ent. faecalis | E. coli | ||
| Chloroform | 100 |
6.20±0.13 Fa |
4.40±0.18 Gb |
0.00±0.00 Fc |
| 150 |
7.00±0.12 Ea |
5.30±0.16 Fb |
3.90±0.04 Ec |
|
| 200 |
8.30±0.14 Ca |
6.00±0.21 Eb |
4.30±0.10 DEc |
|
|
Methanol |
100 |
7.50±0.07 Da |
6.50±0.08 Db |
4.00±0.15 Dc |
| 150 |
8.20±0.10 Ca |
7.00±0.09 Cb |
5.20±0.05 Cc |
|
| 200 |
9.60±0.03 Ba |
8.00±0.06 Bb |
6.20±0.03 Bc |
|
| Ampicillin | 0.2 |
22.0±0.51 Aa |
20.20±0.05 Ab |
17.8±0.03 Ac |
LSD=0.43
The different capital letters refer to significant differences between different groups (concentrations) at (P<0.05)
The different small letters refer to significant differences between different bacteria species at (P<0.05).
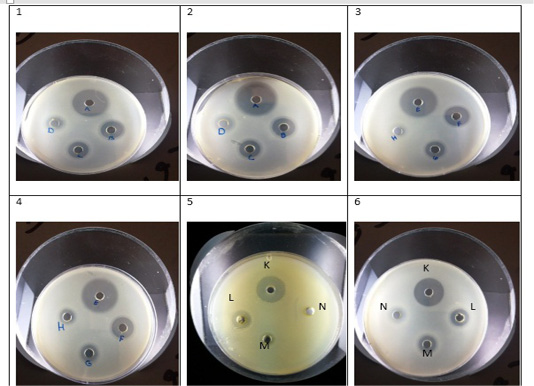 Figure 1: 1- Chloroform extract against Strep. Pyogenes (A- ampicillin, B- 200µg/µl,C- 150µg/µl, D- 100µg/µl. 2- Methanol extract against Strep. Pyogenes (A- ampicillin, B- 200µg/µl, C- 150µg/µl, D- 100µg/µl. 3-Chloroform extract against Ent. Faecalis (E- ampicillin, F- 200µg/µl, G- 150µg/µl, H- 100µg/µl. 4- Methanol extract against Ent. Faecalis (E- ampicillin, F- 200µg/µl, G- 150µg/µl, H- 100µg/µl. 5- Chloroform extract against E. coli (K- ampicillin, L- 200 µg/µl, M-150µg/µl, N- 100µg/µl. 6-Methanol extract against E. coli (K- ampicillin, L- 200 µg/µl, M-150µg/µl, N- 100µg/µl.
Figure 1: 1- Chloroform extract against Strep. Pyogenes (A- ampicillin, B- 200µg/µl,C- 150µg/µl, D- 100µg/µl. 2- Methanol extract against Strep. Pyogenes (A- ampicillin, B- 200µg/µl, C- 150µg/µl, D- 100µg/µl. 3-Chloroform extract against Ent. Faecalis (E- ampicillin, F- 200µg/µl, G- 150µg/µl, H- 100µg/µl. 4- Methanol extract against Ent. Faecalis (E- ampicillin, F- 200µg/µl, G- 150µg/µl, H- 100µg/µl. 5- Chloroform extract against E. coli (K- ampicillin, L- 200 µg/µl, M-150µg/µl, N- 100µg/µl. 6-Methanol extract against E. coli (K- ampicillin, L- 200 µg/µl, M-150µg/µl, N- 100µg/µl.
The antibacterial activity of Cinnamomum zeylanicum extract may be due to presence of active compounds like cinnamaldehyde, alkaloids, tannins, terpenes and saponins which may act synergistically in inhibition of bacterial growth, where the cinnamaldehyde interferes with electron transfers and reactions with nitrogen-containing compounds, resulting in bacterial growth inhibition (Gupta et al., 2008), while saponins have antibacterial effect by combining with cell membranes to elicit changes in cell morphology leading to cell lysis (Moyo et al., 2012). These components are hydrophobic which lead to partition the lipids of the bacterial cell membrane and mitochondria making them more permeable causing leakage of the important molecules and ions and dying of the bacteria (Rastogi, and Mehrotra, 2002).
Conclusions
The both extracts of Cinnamomum zeylanicum barks (methanol and chloroform) have concentration dependent antibacterial activity against gram-positive and gram-negative bacteria including Streptococcus pyogenes , Enterococcus faecalisand Escherichia coli that mean it has broad-spectrum activity, where the methanol extract was more effective than chloroform extract, and the gram positive bacteria were more susceptible than gram negative bacteria.
Recommendations
Testing another solvents for extraction to know the yield amount and testing another concentrations of the methanolic and chloroform extract with experimenting another pathogenic bacteria especially that resistant to antibacterial agents.
Acknowledgements
The authors are thankful to the College of Veterinary Medicine in University of Baghdad for providing facilities and laboratory to complete this study. A special thanks to Dr. Saad Alrawey, for his help to complete the statistical side in present study. There is no specific fund was received for this study.
Conflict of interest
The authors declare that they have no competing interests.
Authors Contribution
Both authors contributed to the planning and doing research work as study design.
References





