South Asian Journal of Life Sciences
Research Article
Investigation of Seasonal Distribution of Mosquito Species in Nay Pyi Taw Area, Myanmar
Si Thu Aung, Soe Soe Wai, Lat Lat Htun, Saw Bawm*
Department of Pharmacology and Parasitology, University of Veterinary Science, Yezin, Nay Pyi Taw 15013, Myanmar.
Abstract | Mosquitoes are well known as annoying pests and as carriers of disease-causing agents to humans and animals. The entomological study was performed in Nay Pyi Taw Union Territory, Myanmar between March 2016 and February 2017. Mosquitoes were collected in three distinct seasons: summer, rainy and winter by using the terminator photocatalysis mosquito trap. Altogether 1224 female mosquitoes belonging to 7 species of 4 genera were observed. Among the collected mosquitoes, Culex quinquefasciatus (75%) showed the highest abundance followed by Armigeres subalbatus (17%), Culex tritaeniorhynchus (3.1%), Aedes aegypti (3%), Aedes albopictus (1.1%), Anopheles spps (0.6%) and Aedes vexans (0.2%) which is accounted for 100% of all mosquitoes collected. The abundance of mosquitoes was numerically highest both in rainy season and in Pyinmana Township. Maximum number of species were found in Pyinmana (n = 485), followed by Lewe (n = 391) and Tatkon (n = 348).It was found that the abundance of mosquitoes was mathematically highest in July throughout the study period.
Keywords | Mosquitoes, Morphological identification, Seasonal distribution, Myanmar
Editor | Muhammad Nauman Zahid, Quality Operations Laboratory, University of Veterinary and Animal Sciences, Lahore, Pakistan.
Received | February 18, 2018 Accepted | April 13, 2018; Published | May 05, 2018
*Correspondence | Saw Bawm, Department of Pharmacology and Parasitology, University of Veterinary Science, Yezin, Nay Pyi Taw 15013, Myanmar; Email: bestshadow@gmail.com
Citation | Aung ST, Wai SS, Htun LL, Bawm S (2018). Investigation of seasonal distribution of mosquito species in nay pyi taw area, myanmar. S. Asian J. Life Sci. 6(1): 7-13.
DOI | http://dx.doi.org/10.17582/journal.sajls/2018/6.1.7.13
ISSN | 2311–0589
Copyright © 2018 Aung et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Mosquitoes are vectors of a number of transmissible, and life menacing diseases such as malaria, filariasis, dengue fever, yellow fever and most of the arthropod borne viral types of encephalitis (Huda and Banu, 1987; Chowdhury et al., 2000). They are the most worrying agents in both urban and rural areas since distant past and persist till now predominantly in the developing countries and the world in general. Due to the global climate change and deforestation the faunal distribution, vectorial capacity has been changed (Clements, 1992).
There are over 3,000 known species of mosquitoes across the globe (Faiman et al., 2014). Mosquitoes have four distinct developmental stages: egg, larva, pupa, and adult. Immature stages of mosquitoes require water to complete their life cycle. Mosquitoes, as all insects, are cold-blooded (poikilothermic) animals and, therefore, are highly dependent on temperature for development and survival. Their growth rate and other aspects of their physiology are temperature-dependent. As the temperature increases, their development time shortens (Silberbush et al., 2005).
Many species of mosquitoes have narrow host preferences. Some prefer to feed only on birds or mammals, or cold-blooded vertebrates such as reptiles and frogs. Consequently, various mosquito species use a wide variety of cues to find a suitable host, often involving a variety of complex interactions that are optimal for their host preference (Smallegange et al., 2012). The distance that mosquitoes can fly is difficult to generalize because mosquito species vary in their flight range. Some species can fly long distances (up to 10km), but most are weak fliers, and do not travel more than several meters (Foster and Walker, 2002; Service, 1997).
Adult mosquitoes are characterized by having long, slender, needle-like mouthparts (proboscis), antennae, and legs. Their narrow wings are often covered with minute scales, and are characteristically folded over their abdomen at rest. Fine scales, bristles and setae cover the mosquito body and vary in coloration from white, silver, or gold, to very dark. Pattern arrangement and scale coloration, among other traits, are often useful for identifying mosquito species (Faiman et al., 2014).
In this study, Nay Pyi Taw served as the main study area, with sampling sites from the three townships. There is a severe nuisance from mosquitoes in many parts of Nay Pyi Taw according to inhabitants complains and relevant control programs are conducted every year. The findings of this study can provide baseline information and act as a starting point for further vector studies because humans have been affected by mosquitoes both as nuisance and as vectors of mosquito-borne diseases for centuries, resulting in economic loss and human suffering (Becker et al., 2010). As a result, it is pivotal to know the status and distribution of mosquito fauna to control mosquito and mosquito borne diseases. The aim of the present study was to determine the seasonal abundance of mosquitoes species around the study area.
MATERIALS AND METHODS
Study Area
The study was performed in Nay Pyi Taw Union Territory, Myanmar, located between Bago Mountain Range and Shan Mountain Range (19°45´ N 96° 6´ E ; MST UTC + 06 : 30 with average temperature of 21.1°C ~ 32.5°C and precipitation 45.33 inches). It was carried out in three townships: Pyinmana (Eastern part), Lewe (Southern part) and Tatkon (Northern part). Location map of study area is described in Figure 1.
Mosquito Collection and Identification
Adult mosquitoes were collected by using the “terminator” photocatalysis mosquito trap. Trap sites were set up at two places in each township. Collecting mosquitoes was carried out throughout a year (from March 2016 to February 2017) corresponding to the different seasons: summer (March, April and May), rainy (June, July, August, September and October) and winter (November, December, January and February). Traps were operated between the hours of dusk and dawn (at 18:00- 7:00). Trapping was performed at first and last week of every month per trap site. The collecting mosquitoes at each trap location was held separately and transported to the laboratory. Male mosquitoes were rejected and only female mosquitoes were kept into tubes containing silica which was labelled by species, trap location and date respectively.
Mosquitos’ identification was performed based on morphologic keys as described in Ramalingam (1976), Huang (1977) and Russell (1996).
Morphological Keys for Identification of Mosquito Species
All mosquitoes specimens were examined based on their morphological characteristics and identified to the species level. Collected mosquitoes were morphologically identified using the following taxonomic keys.
Adult Culex quinquefasciatus is a medium-sized mosquito and is brown in colour. The body is about 3.96 to 4.25mm long. While the main body is brown, the proboscis, thorax, wings, and tarsi are darker than the rest of the body. The head is light brown with the lightest portion in the center. The antennae and the proboscis are about the same length, but in some cases, the antennae are slightly shorter than the proboscis. The flagellum has 13 segments that may have few or no scales. The scales of the thorax are narrow and curved. The abdomen has pale, narrow, rounded bands on the basal side of each tergite. Males can be differentiated from females in having large palps and feathery antennae (Australian wildlife; www.OzAnimals.com).
Culex tritaeniorhynchus is a relatively small, reddish brown species (Becker et al., 2010). It can be identified by the dark brown scaling on the vertex and scutum, the accessory pale patches basal to the pale band on the ventral surface of the proboscis, and the narrow apical dark ring on the hind femur (Reuben, 1994).
Aedes aegypti is a small, dark mosquito with conspicuous white markings and banded legs, a black proboscis and white scaling on the tips of the palps (Russell 1993).
Aedes albopictus is characterized by its black-and-white-striped legs, and small black-and-white-striped body. The insect is called a tiger mosquito for its striped appearance, which resembles that of the tiger. A single silvery-white line of tight scales begins between the eyes and continues down the dorsal side of the thorax. This characteristic marking is the easiest and surest way to identify the Asian tiger mosquito. The proboscis is dark colored, the upper surface of the end segment of the palps is covered in silvery scales, and the labium does not feature a light line on its underside. The compound eyes are distinctly separated from one another. The scute, the dorsal portion of an insect’s thoracic segment, is black alongside the characteristic white midline. On the side of the thorax, the scutellum, and the abdomen are numerous spots covered in white-silvery scales. Such white-silvery scales can also be found on the tarsus, particularly on the hind legs that are commonly suspended in the air. The femur of each leg is also black with white scales on the end of the “knee” (Huang, 1968).
Table 1: Combined the overall abundance, the seasonal distribution and abundance as well as localized abundance of mosquitoes in three townships
| Species | Cx. quiquefasciatus | Cx.tritaeniorhynchus | Ae.aegypti | Ae.albopictus | Ae.vexans | Ar.subalbatus |
Anophele spp. |
||
| Summer | Mar | Tatkon | 16 | 1 | 6 | ||||
| Pyinmana | 27 | ||||||||
| Lewe | 29 | 1 | 1 | ||||||
| Apr | Tatkon | 15 | 5 | 2 | 5 | ||||
| Pyinmana | 24 | 2 | |||||||
| Lewe | 20 | 11 | |||||||
| May | Tatkon | 16 | 1 | ||||||
| Pyinmana | 19 | 2 | |||||||
| Lewe | 17 | 3 | |||||||
|
Rainy* |
June | Tatkon | 9 | 1 | 10 | 5 | 2 | ||
| Pyinmana | 12 | 3 | 6 | ||||||
| Lewe | 13 | 5 | 3 | ||||||
| July | Tatkon | 45 | 2 | 1 | 2 | 1 | |||
| Pyinmana | 101 | 3 | 5 | ||||||
| Lewe | 60 | 3 | 8 | ||||||
| Aug | Tatkon | 11 | 1 | 2 | 10 | 2 | |||
| Pyinmana | 43 | 3 | 2 | 2 | |||||
| Lewe | 42 | 2 | 1 | ||||||
| Sep | Tatkon | 6 | 1 | 22 | 1 | ||||
| Pyinmana | 9 | 3 | 1 | 21 | |||||
| Lewe | 19 | 7 | |||||||
| Oct | Tatkon | 7 | 1 | 1 | 13 | ||||
| Pyinmana | 35 | 8 | 1 | ||||||
| Lewe | 11 | 2 | 9 | ||||||
| Winter | Nov | Tatkon | 18 | 1 | 9 | ||||
| Pyinmana | 41 | 1 | 26 | ||||||
| Lewe | 20 | 1 | 9 | ||||||
| Dec | Tatkon | 28 | 3 | 2 | |||||
| Pyinmana | 24 | 6 | |||||||
| Lewe | 20 | 10 | 2 | ||||||
| Jan | Tatkon | 24 | 8 | ||||||
| Feb | Tatkon | 32 | |||||||
| Lewe | 23 | 10 | |||||||
| Feb | Tatkon | 32 | |||||||
| Pyinmana | 22 | ||||||||
| Lewe | 28 | 1 | |||||||
| Total | 918 | 38 | 37 | 14 | 3 | 207 | 7 | ||
*(p<0.05)
Adult Aedes vexans females are medium sized with a dark proboscis and dark femura. The tarsi are banded and the pronotum shows curved yellow scales, mesonotum clothed with bronze scales, wing scales brownish black with white scales at the base (Gjullin and Eddy, 1972). The first tergite has dark and pale scales intermixed, tergites II-VI are dark with basal white band and basolateral patches (Carpenter and Casse, 1955).
Anopheles mosquitoes can be distinguished from other mosquitoes by the palps, which are as long as the proboscis, and by the presence of discrete blocks of black and white scales on the wings. Adults can also be identified by their typical resting position: males and females rest with their abdomens sticking up in the air rather than parallel to the surface on which they are resting (Centers for Disease Control and Prevention).
Armigeres adults are morphologically similar to species of other aedine generic-level taxa in the Oriental and Australasian Regions but they are generally larger and usually have the proboscis slightly curved downwards and flattened laterally. They are more reliably distinguished from other aedine genera with the following combination of characters: dorsal head scales mostly broad and flat; compound eyes separated ventrally by two long rows of scales; acrostichal setae, dorsocentral setae and prespiracular setae absent; postspiracular scales present; postspiracular setae present in subgenus Armigeres, mesomeron small with base more or less in line with base of hindcoxa; alula and upper calypter of wing with hair-like scales on margin (Mosquito Taxonomic Inventory; http://mosquito-taxonomic-inventory.info/simpletaxonomy/term /6074).
Statistical Analysis
Data was collected and entered by using the Excel program and the statistical analysis was done by using Statistical Package for the Social Sciences (SPSS version 16.0) for the windows (SPSS Inc., Chicago, IL). Significant differences in mosquitoes abundance by month, seasonal abundance of mosquitoes and localized abundance of mosquitoes were tested by one-way analysis of variance (ANOVA) ‘F’ test. The level of statistical differences were used for p<0.05.
RESULTS
A total of 1224 female mosquitoes belonging to four genera (Anopheles, Aedes, Culex and Armigeres) were captured. Amidst them, 7 species of mosquitoes were identified with morphological keys. Female mosquitoes were collected in three townships in Nay Pyi Taw Union Territory throughout a year albopictus (from March 2016 to February 2017) in connection with the different seasons: summer, rainy and winter seasons. A total of 918 Cx. quinquefasciatus, 207 Ar. subalbatus, 38 Cx. tritaeniorhynchus, 37 Ae. aegypti, 14 Ae. albopictus, 3 Ae. vexans and 7 Anopheles species were observed in three townships throughout the year.
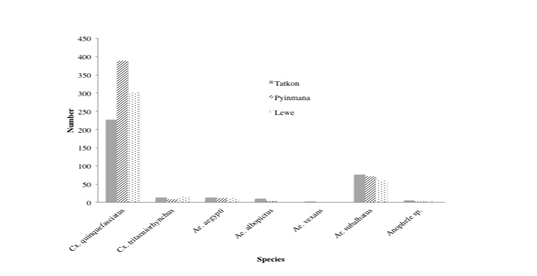
Numbers of the Cx. quinquefasciatus (75%) were the most frequently collected mosquito, followed by Ar. subalbatus (17%), Cx. tritaeniorhynchus (3.1%), Ae. aegypti (3%), Ae. albopictus (1.1%), Anopheles spps (0.6%), and Ae.vexans (0.2%), which is accounted for 100% of all mosquitoes collected. The percentage of Cx. qionquefasciatus was mathematically highest not only in all seasons but also in three townships and was the most common species during the study period. The abundance of mosquitoes was numerically highest both in rainy season and in Pyinmana Township. It was found that the abundance of mosquitoes was mathematically highest in July throughout the study period. When identifying the species of mosquitoes in study area, the overall abundance, the seasonal distribution and abundance as well as localized abundance of mosquitoes are shown in Table 1 and Figure 2.
According to ANOVA ‘F’ test, there was not a significant difference within the three townships. However, the number of mosquitoes was mathematically highest in Pyinmana (485), followed by Lewe (391) and Tatkon (348).
Overall, there was no significant difference when the numbers of mosquito species were compared among the three seasons. However, significant differences between rainy and summer season were observed (p<0.05). The summer and winter as well as the rainy and winter were not significantly different in three seasons.
Figure 1: Map of study area.
DISCUSSION
Mosquitoes are ubiquitous insects, they can be found in nearly every type of climatic region of the world from the arctic regions to the tropics, surviving severe winters or dry seasons depending on the region. Depending on species they can be found breeding in all types of water; from heavily polluted, to clean; from small collections of water in tin cans, to pools or streams; such is their adaptability. Their distribution is increased and aided by transport systems such as boats and aircraft and non-indigenous species have been introduced to new territories in this way, even infected mosquitoes have been transported to temperate climates, thereby transmitting tropical diseases. Despite research and decades of mosquito control efforts all over the world, mosquitoes remain a major public health problem. The data for the mosquito vectors and their seasonal is rare in Myanmar even though the survey of mosquito-borne disease had been reported. Thus, the present study was performed to reveal what mosquito species distribute and their seasonal occurrence in Nay Pyi Taw area.
Culex quinquefasciatus is one of the most widespread mosquitoes in the world. It is found throughout most of pan and subtropical Americas (Barr, 1957), the Neotropics, Indomalayan, Australasian and Eastern Asian regions of the world (Lee et al., 1989). It is also present in the United Kingdom and parts of the Middle East. In the present study, numbers of the Cx. quinquefasciatus (75%) were the most frequently collected mosquito, followed by Ar. subalbatus (17%), Cx. tritaeniorhynchus (3.1%), Ae. aegypti (3%), Ae. albopictus (1.1%), Anopheles species (0.6%), and Ae. vexans (0.2%), which is accounted for 100% of all mosquitoes collected.
Culex quinquefasciatus Say, 1823 (originally named Culex fatigans), commonly known as the sorthern house mosquito, was found with the highest percentage amidst the mosquito species collected in the present study. Many studies of Cancrini et al. (2007), Dyab et al. (2015) and Karim (2013) described that the highest percentage of Cx. quinquefasciatus in their study areas were 70.3% in Italy, 69% in Egypt and 29% in Bangladesh. However, it had not been recorded in Portugal (Ferreira et al., 2015). Culex species Lay egg in masses called rafts although Anopheles species and Aedes species Lay eggs singly. For mosquitoes that Lay eggs in clusters or rafts, a standard egg raft is about ¼ inch (6.35 mm) long and contains 100-200 eggs. Egg rafts cannot withstand desiccation and are usually associated with permanent or semi-permanent water bodies. They will hatch after about two days on the water and without constant water, they desiccate and die (Silberbush et al., 2005) In this study, it was found out that there are a lot of mosquito breeding places for Cx. quinquefasciatus like polluted surface waters or artificial containers, shallow ponds within streams, artificial habitats such as drains, stock drinking troughs, septic tanks, rain water containers, tyres and various other small containers in the study areas. It is assumed that eggs in single are more probable to degrade eggs in clusters or rafts because of the climatic factors and environmental conditions. As a result, it could be explained that it is impossible to desiccate the egg due to the favouring the abundance of Cx. quinquefasciatus and they may become very numerous in the study areas.
In this study, the seasonal occurrence of mosquitoes was performed to provide baseline data of the mosquito species, to be awareness of species imposing risk to public health and to assist mosquito control program. In the present study areas, according to the meteorological station of Nay Pyi Taw, the mean temperature were 31.5°C in summer, 28.01°C in rainy and 24.31°C in winter seasons. The rainfalls in each season were 33.2 inches in summer, 103.2 inches in summer, 103.2 inches in rainy and 27.48 inches in winter seasons. This weather conditions favorable for growth and activities of mosquitoes and increased mosquito population in this study areas.
The present study revealed that the occurrence of mosquitoes was the highest in July amongst the other months throughout the study period in all study areas. The rainfalls and the mean temperature in July were 55.6 inches and 27.9°C. It is assumed that this weather condition in July is more likely to increase mosquito population than among the other months. Moreover, it is found that July is the highest significant amidst the months throughout the year.
According to location, the present study revealed that the occurrence of mosquitoes was the highest in Pyinmana (39.62%) followed by Lewe (31.94%) and the lowest in Tatkon (28.43%). It could be explained that there was small difference in temperature and rainfall which favour for mosquito breeding among the study areas as these three townships in Nay Pyi Taw are very close one after another.
In seasonal abundance of mosquitoes, the percentage of mosquito was observed highest in rainy season (48.94%), followed by winter (32.84%) and summer (18.22%) seasons. It was investigated that rainy season lasts five months, the winter season has four months and the last one, summer stands three months in Myanmar seasons and therefore sample collection time is the longest in rainy season and stands second in winter. Furthermore, the rainy season has more favourable conditions such as climate factors, having constant water, rain water containers, swamps, grass, small collections of ground water and rice fields that profusely promote for mosquito breeding than the other as well as the mean temperature and rainfall in winter season during this study period were 24.31°C and 27.48 inches. As the geographical distribution of a species in a given area without absolute barrier to dispersal, might be determined by environmental variations such as temperature and humidity (Bates, 1949; Samways, 1995; Micieli, 2003). As a result, it was assumed that these temperature, rainfall and environmental conditions were favourable for mosquito breeding in the present study. Moreover, Cx. quinquefasciatus (75%) was the most frequently collected mosquito and was also observed all the year round in each study area. Mature Cx. quinquefasciatus females fly at night to nutrient-rich standing water to lay eggs. The larvae feed on organic material in the water and require five and eight days to complete their developments at 30°C. After 36 hours at 27°C, adults emerge. A single female can lay up to five rafts of eggs in a lifetime, with each raft containing thousands of eggs. In optimum temperature and humidity, the lifecycle will be completed in seven days, passing through the egg, larval, pupal and adult stages (Hill et al., 2009). Once hatched the larvae are able to overwinter in the cooler months of July to September, while adult activity ceases (Lee et al., 1989, Weinstein et al., 1997). During the study period of Nay Pyi Taw area, the mean temperature, rainfalls and bionomics in rainy season particularly in July and August are very favourable to profusely breed Cx. quinquefasciatus females fly at night and therefore it could be explained that Cx. quinquefasciatus is the most abundant mosquito species in the present study because mosquito traps were operated between the hours of dusk and dawn (at 18:00pm – 7:00am throughout a year).
In comparison of number of Cx. quinquefasciatus observed by location, it was investigated that Pyinmana was mathematically higher than Tatkon. It could be deduced that mosquito collection sites in Pyinmana were more crowded, feeable in rarely drainage system and weak in knowledge about mosquito control methods than that of Tatkon.
Ar. subalbatus was the second common species found in the present study and mostly found in rainy season and it was found to be prevalent in three townships of Nay Pyi Taw throughout the study period. However, the other five species were scare in the present study. The population trends shown by the various mosquito species during this study may provisionally be taken as a guide to their seasonal variations. It was not easy to say what factors were critical for the abundance or scarcity of particular species in a given area, since the answer demands critical investigation of the factors affecting a population and such studies were not undertaken during the present work.
In the present study, morphological identification was carried out on 1224 adult female mosquitoes collected in Nay Pyi Taw area. Morphological identification of specimens is time-consuming requires specialist knowledge and can be problematic when trying to identify damaged specimens or distinguish morphologically similar species (Batovska et al., 2016). Molecular identification of mosquitoes should be conducted because PCR was the more sensitive technique for the confirmation of mosquito species genome.
Hence, the current study provides useful information about the abundance of mosquito species in the three townships of Nay Pyi Taw. It is also essential to extend the mosquito- borne diseases and to be awareness of an appropriate mosquito control management.
ACKNOWLEDGEMENTS
Authors would like to express their sincere gratitude to Professor Dr. Yuki Eshita, Zambia Project, Division of International Collaboration and Education, Hokkaido University Research Center for Zoonosis Control for his kind help and guidance during species identification. We are also special thanks to all the staffs of Laboratory of Parasitology Department of Disease Control, Graduate School of Veterinary Medicine, Hokkaido University, Japan for providing necessary aid throughout the research work and Department of Pharmacology and Parasitology, University of Veterinary Science for contributing the collection of mosquitoes.
CONFLICT OF INTEREST
The authors declare that they have no competing interest.
AUTHORS CONTRIBUTION
All authors contributed equally in research and writing of manuscript.
REFERENCES