South Asian Journal of Life Sciences
Research Article
Eco Biology and Life Cycle of the Pea Blue Butterfly, Lampides boeticus (Linnaeus) (Lepidoptera: Rhopalocera: Lycaenidae) from Southern Andhra Pradesh, India
Harinath Palem, Suryanarayana Kanike, Venkata Ramana Sri Purushottam*
Department of Zoology, Yogi Vemana University, Y. S. R Kadapa District- 51600, Andhra Pradesh, India.
Abstract | Life history of the long tailed blue butterfly also called pea blue, Lampides boeticus (Linnaeus) with respect to larval stages, diet consumption, exploitation and the length of lifae phase was designed to perform. The study was conducted between January to December 2014 at Yogi Vemana University Campus (78°42’46.429’ E, 14°28’10.542’ N) and Campus Botanical garden (78°42’44.539’ E, 14°28’25.59’ N), Kadapa, India. Lampides boeticus completes its life cycle in 15-22 days (Eggs 2-3 days, Larvae: 8-12days, Pre-Pupa:2-3days, Pupa: 3-4 days). The standards of food indices across the instars include Approximate Digestibility (AD): 90 – 96.07%; Growth Rate (GR): 0.51 - 0.64, Consumption index (CI): 11.54 - 5.95, Efficiency of Conversion of Digested food (FCD): 03.98 – 11.25%; Efficiency of Conversion of Ingested food (ECD): 03.85 – 10.80% as measured in the research laboratory. The relatively high values of ECD and ECI partially explain the ecological success of this butterfly in the present study enviroment.
Keywords | Life history, Lampides boeticus, Vigna trilobata (L.), Food indices, Yogi Vemana University Campus, Sothern Andhra Pradesh
Editor | Muhammad Nauman Zahid, Quality Operations Laboratory, University of Veterinary and Animal Sciences, Lahore, Pakistan.
Received | August 08, 2015; Revised | October 04, 2015; Accepted | October 05, 2015; Published | October 24, 2015
*Correspondence | Venkata Ramana Sri, Yogi Vemana University, Vemanapuram, Kadapa, Y. S. R Kadapa, Andhra Pradesh, India; Email: akshay@yogivemanauniversity.ac.in
Citation | Palem H, Kanike S, Purushottam VRS (2015). Eco biology and life cycle of the pea blue butterfly, Lampides boeticus (Linnaeus) (Lepidoptera: Rhopalocera: Lycaenidae) from Southern Andhra Pradesh, India. S. Asian J. Life Sci. 3(1): 14-21.
DOI | http://dx.doi.org/10.14737/journal.sajls/2015/3.1.14.21
ISSN | 2311–0589
Copyright © 2015 Palem et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Butterflies are a vital part of the ecological system. They play a vital role as pollinators and since they are migratory on this count on the top of conservation biology agenda. Insects and plants are the dominant groups among the various life forms of the planet earth. The migrations between the two groups are largely responsible for their dominance and diversification. One of the insect groups; the butterflies also add immense aesthetic value to environment and contribute to the genetic diversity in angiosperms through mediating cross pollination. However, these insects suffered losses and declines in the pest decades all over the world due to habitat loss. In India butterfly trade is another major factor for the decline of the butterfly population. Due to worldwide pressure on natural biomes, butterflies have previously been shown to be extremely sensitive indicator of temperature change (Harinath et al., 2015, Kehimkar, 2008), biotope disintegration (Atluri et al., 2002d) and development (Atluri et al., 2004). The situation may be more serious among the endemic species. As a valuable component of the ecosystem, the survival of butterflies and their population’s density must be improved through appropriate and effective conservation measures (Harris and Harris 1997; Withgott 1999). The need for effective conservation strategies was urgent in view of the accelerated destruction of natural niche causing disturbance in the butterfly’s habitat. One of the measures often suggested is the captive breeding and subsequent release of butterflies in the wild (Harinath et al., 2015). The butterfly provides a quick indication for habitat quality and are also sensitive indicators of climate change (New et al., 1995; Venkata et al., 2003). The success of such efforts requires sound knowledge of the biology and ecology of butterflies.
The present study explains the details of nectar resources for adults, life history stages, population index and food utilization efficiencies of different larval stages of the Pea blue Lampides Boeticus. The life history of the long tailed blue butterfly also called pea blue, Lampides boeticus (Linnaeus) larval stages in relations to diet consumption, exploitation and the length of life phase on its host plant Vigna trilobata (L.) have been designated for the first time. The pea blue, Lampides boeticus was one of the species of Lycaenidae butterfly (classification) originated in the Palaeotropics (Figure 1), and it was usually seen in exposed scrub and grassland environments.
MATERIALS AND METHODS
Study Area
The present study was carried out during January 2014 to December 2014, in the campus botanical garden (78°42’44.539’ E, 14°28’25.59’ N) and Yogi Vemana University Campus, Kadapa. (78°42’46.429’ E, 14°28’10.542’ N) (Figure 2), the botanical garden was 1 km from the University campus and vegetation consists mainly of the deciduous scrub jungle type. The university campus hosts good vegetation, both wild and cultivated including shrubs, trees and weeds.
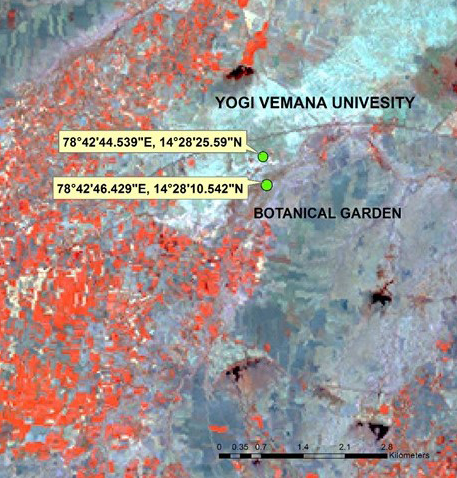
Figure 2: Study Area
Both sites were regularly searched for the reproductive activity of the Pea Blue butterfly. Once located, detailed observations were made in order to record the period of copulation and oviposition, followed by fresh egg collection without causing any damage to the larva. After oviposition, the leaf with eggs was collected into Petri dishes (15 cm × 2.5 cm depth) and transported to the research laboratory. The piece of the leaf with the eggs was then placed in a small Petri dish (10 cm × 1.5 cm depth), the inside of which was lined with moistened blotter to prevent the leaf from drying. Such Petri dishes were kept in hygienic, spacious cages fitted with wire gauge. Since ants were never detected, no special protection device was used to stop predation of eggs. They were inspected regularly at 6 h intervals to record the time of hatching. Each of the freshly emerged larvae was transferred to a clean Petri dish the inside of which was lined with moistened blotter with the help of a camel hair brush.
The larvae were provided daily with a weighed amount of leaf pieces of the host plant. The faeces and the leftover of the foodstuff were collected and weighed each day (24 h). The growing larvae were observed regularly to note the instar change and features including length and weight measurements. As the larvae grew, they needed more space, increased space was provided by transferring the growing larvae to bigger Petri dishes (15 cm × 2.5 cm depth). Larval development in terms of food consumption indices were designed by Waldbauer (1968) as given in Figure 3.
RESULTS AND DISCUSSION
Description
Field characters
In wing tip spread, measurment is 24 - 34mm (male & female). Body was slender and short: 10.0- 12.0 (11.0-0.08) mm long, dark brown on dorsal and white on ventral side (Figure 4).
Male and female are distinguishable by their colouration on their dorsal side, the former being violet blue and the latter brown in both cases with a blue colouration at the

Figure 4: Pattern of wings of Pea Blue butterfly
wing bases. On the ventral side both the sexes have pale brown to white traversed or light to reddish brown narrow bands on both hind wings and forewings. Such bands are absent and distinct white band is present within the outer margin of hind wings. In both sexes hind wing show up at their forms region two below. Each hind wing has a delicate, black and white tipped tail.
Habitats
It was observed to be an efficient flier and it flies fast with rapid wing beats. It could cover long distances in a single flight. It moves about in bushes searching for nectar. Both sexes used to bask with their wings half open. It was often found sitting on the walls on the verandas of buildings in the university campus.
Flight Period in Eastern Ghats
Winged butterflies were observed throughout the year, contingent on the area and whether its food plants are in flowering or not. In the far Eastern Ghat zones the butterfly was largely dependent on the rainy periods. In the southern areas of native vegetation it was seen more commonly during the spring, while in the metropolitan garden it was found during the warmer months. They are capable of finishing a brood within about seven weeks in southern areas. In the cold areas the butterflies generally hibernate as pupae.
Distribution
Found throughout the year in Asian countries like India, Pakistan, Afghanistan, Nepal, Bhutan and Sri Lanka. It has relocation tendencies, which are intermittently witnessed in India, heading in a southerly direction. This is supported by the adult morphology, which was uniform across India, and also the rest of the Eastern Ghats where the butterfly also is commonly seen. No mass migrations have ever been reported from Eastern Ghats of Southern India.
Management Standing in Eastern Ghats
Occurs locally in breeding areas. Occasionally common in the Western Ghats areas after rains when there may be a uniform mass blooming of its food plants, rarely common in the Eastern Ghats areas.
Threats
Adult butterflies face threat from natural predators like birds, lizards and spiders. The organisms that destroy butterflies in different stages of their life were divided into three main categories, based on the way they feed upon the butterflies 1. Parasitoids; 2. Parasites and 3. Predators
Conservation Strategy
Conservation strategy of Lampides boeticus - Lycaenidae is listed in The Indian Wildlife (Protection) Act, 1972. Schedule II, Part II. This butterfly sometimes considered as pest due to the fondness of its larvae for low growing leguminous crops and vegetables. Presently there are no special polices for conservation of insects in the wildlife Sanctuaries and National parks of India. However, the control of fires and grazing in the protected areas may be the best strategy that forest departments can follow to maintain the diversity of butterflies and insects.
Nectar Plants
Although the males and females of the pea blue butterfly Lampides boeticus foraged on several plant species in flower at the study sites, 20 plant species were identified as the preferred ones and their flowering periods were recorded (Table 1).
|
S.No |
Plant species |
Flowering period |
|
1 |
Hibiscus rosasynensis L. |
Throughout year |
|
2 |
Zizyphus mauritiana Lamk. |
Aug to Oct. |
|
3 |
Z. oenoplia Mill |
Aug to Oct |
|
4 |
Z. xylopyrus Wild |
Jun to Aug |
|
5 |
Pongamia glabra Vent. |
Apr to Jun |
|
6 |
Tridax procumbens L. |
Throughout year |
|
7 |
Jasminum angustifolium. Wild. |
Jun to Aug |
|
8 |
Nerium odorum L. |
Throughout year |
|
9 |
Lantana camara L. |
Throughout year |
|
10 |
Duranta repens L. |
Jun to Dec |
|
11 |
Ficus religiosa |
May to Aug |
|
12 |
Ficusbenghalensis |
May to Aug |
|
13 |
Citrus lemon |
April to July |
|
14 |
Mangifera indica |
March to Jun |
|
15 |
Calotropis gigantia |
Throughout the year |
|
16 |
Calotropis procera |
Throughout the year |
|
17 |
Cassia alata |
Jul to Dec |
|
18 |
Cassia siamea |
Jul to Dec |
|
19 |
Cassia fistula |
Jul to Dec |
|
20 |
Cassia occidentalis |
Jul to Dec |
Table 2: Duration of different stages of Lampides boeticus during different seasons of of the year Vigna trilobata (L.)
|
Season |
Egg (Days) |
Ist Instar |
IInd Instar |
IIIrd Instar |
IVth Instar |
Pre-Pupa |
Pupa |
Total life cycle duration (Days) |
|
Pre Monsoon (May-June) |
2-3 |
2-3 |
2-3 |
2-3 |
2-3 |
2-3 |
3-4 |
15-22 |
|
Post Monsoon (Oct-Nov) |
3-4 |
3- 4 |
3-4 |
3-4 |
3-4 |
3-4 |
4-5 |
22-29 |
Table 3a: Morphometric measurements of different stages of Lampides boeticus during season of the year Vigna trilobata (L.)
|
Egg |
Ist instar |
IInd Instar |
IIIrd Instar |
IVth Instar |
Pupa |
|
0.6-0.7 mm |
1.0 – 1.3 mm |
2.0-3.0 mm |
3.0 – 3.5 mm |
5.0-8.0 mm |
10.0-11.0 mm |
Table 3b: Population index of eggs of Lampides boticus on Vigna trilobata (L.) buds in the filed
|
Calendar month |
Adult abundance |
Number of eggs |
|
May Jun July August September October November December January February March April |
Common Common Common Very Common Very Common Very Common Very Common Very Common Common Common Common Common |
06 08 11 16 27 13 14 11 7 6 4 3 |
Ovipostion Host Plants
Although the pea blue butterfly is known to feed on many febaceae plants including several crotalaria spp, present study found Vigna trilobata (L.) as the most desirable plant for oviposition among this butterfly population.
Egg Stage
Mating occurred during day time and lasted for an hour. The gravid female placed eggs singly on the sepals of the flower buds besides stalks through most of the day time , but more frequently during 1100- 1600 hr about 1-5 eggs were deposited on each flower bud. They were greenish blue in colour, which turned pale before hatching. They were turban shaped, flat on top and at bottom, depressed in the centre of the top; surface was covered with polygonal cells. They measured 0.6 – 0.7 (0.66- 0.40) mm in diameter. Hatching occurred within 2-3 days of incubation. The enclosed larva did not feed on its inner contents. It moulted four times and appeared in four instars over a period of its development (Table 3a).
Larval Stage
Instar I (c)
The 1st instar larvae after hatching, they are pale creamy in colour, this stage lasted for 2-3 days, attained a length of 1.0 -1.3 mm and a width of 0.45 – 0.65 mm. The larva in this stage was not clearly visible to the naked eye. The duration of the first instar varied from 2-3 days during Pre Monsoon (May-June) and 3-4 days during Post Monsoon (October- November) (Table 2) .
Instar II (d)
This stage also lasted for 2-3 days, attained a length of 2.0 - 3.0 (2.50 ± 0.40) mm and a width of 0.70 - 0.90 (0.80 ± 0.08) mm. It was brown, with a mid- dorsal dark reddish brown line. Its head was black. The duration of the second instar varied from 2-3 days during Pre Monsoon (May-June) to 3-4 days during Post Monsoon (October- November) (Table 2) this variation continued up to IV instar.
Instar III (e)
This stage too lasted for 2-3 days. It reached a length of 3.00 – 3.50 (3.30 ± 0.21) mm and a width of 1.00 – 1.50 (1.26 ± 0.20) mm. the larva was clearly visible in this stage. It was woodlouse shaped greenish brown hairy with mid – dorsal reddish brown line appears.
Instar IV (f & g)
This stage lasted for 2-3 days, grew to 5.00 – 8.00 (6.33 ± 1.24) mm in size and 1.80 – 2.50 (2.10 ± 0.94) mm in thickness. It was lemon yellow to pale green in colour, with clear segmentation. Black spots (spiracles) appeared laterally on each segment. There was no change in other characters recorded in the third instar.
Pupal Stage
Pre Pupa (h)
This stage lasted for 2-3 days. The full grown larva stopped feeding and got shortened measuring 10mm in length and 5mm in width. It was pale brown in colour. Pupation in the field took place at the bases of stems or among fallen leaves or flowers on the ground.
Pupa (i)
The pupal stage lasted for 3-4 days. Anal end was fairly broad. Thorax was slightly compressed. Abdomen was broader than the breadth at shoulders. Pupal body was humped in the middle. It was dorsally brown in colour with a dark brown dorsal line. On both sides of this dorsal line dark brown spots and blotches were present. Ventrally it was light brown, soft and plain without any markings. It attached itself to the substratum at the middle of its body by a loose body band. It is measured 10.0-11.0 (10.50 – 0.04) mm in length and 3.5 -5.0 (4.16 – 0.62) mm in size at its largest end. The duration of the pupa varied from 4-5 days during Pre Monsoon (May-June) to 3-4 days during Post Monsoon (October- November) (Table 2).
Duration of Life Cycle
It ranged between 15-22 days (Eggs 2- 3, Larvae, 8 – 12, Pre-Pupa-2-3, Pupa 3-4).
Population Index
Only the eggs could be spotted (Table 3b). The larvae are hidden inside the flowers and the pupae are formed in the soil. The numerical frequency of the natural occurrence of the life stages – eggs, larvae, pupae and adult on the host plant are given in Figure 5. The numerical frequency of eggs scored on the searches made on some Vigna trilobata (L.) plants suggested that Lampides boeticus could reproduce throughout the year. The period during August - December recorded a higher frequency of reproduction (Figure 6).
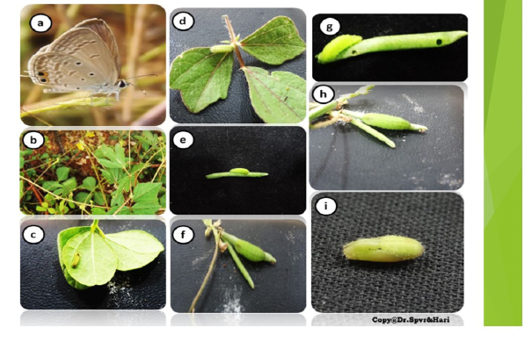
Figure 5: Life stages of Pea Blue butterfly
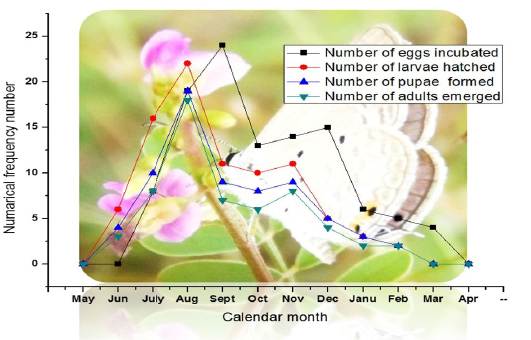
Figure 6: Life stages of Pea Blue butterfly
Food Consumption, Growth and Utilizations
The newly hatched larvae bored in to the buds of the host plant and consumed mostly the inner parts i.e. after eating a flower; it came out and shifted to another flower. The change of one instar to the other in the laboratory was identified by the presence of moulted skin in the Petri dish. Also when the larvae were about to moult, it did not enter the newly supplied flowers and entry was there only after completion of moulting.
Floral nectar was main source of food in the adult stage of butterflies. Lampides boeticus visits blossoms regularly and imbibes nectar, higher nectar intake may increase its longevity and egg production (Fischer and Fiedler 2001; Grill et al., 2013). This butterfly mostly visits the flowers of Tridox procumbens, Duranta repens, Lantana camara, Pongamia glabra and Cassia alta. The total development time from egg laying to adult formation was ~15-22 days.
This developmental lifetime was in line with the expectations of short life cycles in tropical butterflies (Owen 1971). Since temperature influences instar duration and the overall development time (Mathavan and Pandian 1975; Palanichamy et al., 1982; Pathak and Pizvi 2003; Braby 2003), this tendency of greater consumption by the last two instars has been reported in lepidopterous larva in general (Waldbauer 1968; Mathavan and Pandian 1975; Scriber and Slansky 1981; Palanichamy et al., 1982; Selvasundaram 1992; Gosh and Gonchaudhuri 1996) and it compensates for the energy expenditure of non-feeding pupal stage (Pandian, 1973).
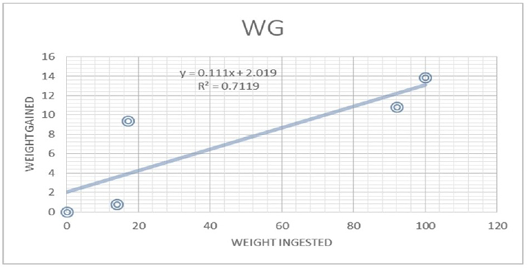
Figure 7: Food comnsumption and weight gain
Table 4 (Figure 7 and 8) gives the data on the amount of food consumed by each of the five instars and the corresponding data on weight gained by different instars. The quantity of food consumed increased from instar to instar. There was a vast increase in the consumption of food from instar I to IV. The weight gain increased from instar I to instar IV. Thus, there was over 92% of the total food consumption and 95% of total weight gained in the IIIrd and IVth instars together. There was a direct relationship between food consumption and growth across the four instars. Regression of weight gained by larva against the food consumed per day showed a straight line relationship between these two variable with r value (r =0.7119) and Y
Table 4: Food consumption, growth and food utilization efficient of lampides boticus fed with Vigna trilobata (L.) buds
|
Instar Number |
Wt. of food ingested (mg) |
Wt. of faeces (mg) |
Wt. gained by larva (mg) |
GR (mg/day) |
CI (mg/day) |
AD (%) |
ECD (%) |
ECI (%) |
|
I |
14.0 ± 01.50 |
4.5 ± 00.78 |
0.75 ± 0.10 |
0.51 |
11.54 |
96.07 |
03.98 |
03.85 |
|
II |
17.0 ± 02.02 |
07.5 ± 0.23 |
9.4 ± 2.78 |
0.39 |
07.94 |
95.05 |
05.12 |
04.90 |
|
III |
92.0 ± 3.16 |
3.9 ± 01.65 |
10.80 ± 1.35 |
0.59 |
05.61 |
92.00 |
10.51 |
09.21 |
|
IV |
100.0 ± 03.30 |
4.0 ± 01.10 |
13.89 ± 1.90 |
0.64 |
05.95 |
90.00 |
11.25 |
10.80 |
GR: Growth rate; CI: Consumption index; AD: Approximate digestibility; ECD: Efficiency of conversion of digested food; ECI: Efficiency of conversion of ingested food (Weight by mean ± SE)
Figure 8: Food consumption and growth rate
value (Y= 0.111+ 2.019) at 0.01 level correlation (Figure 7). The values of growth rate (GR) increased till instar III and then decreased to instar II, and consumption index (CI) progressively decreased from instar to instar. The values of GR varied between 0.39 – 0.64 mg/day and those of CI between 11.54 – 05.95 mg/day. Table 4 also includes the data on AD, ECD, and ECI. The values of AD from instar I to instarIV decreased from a high of 96.07% in first instar to a low of 90.0% in the last instar. The values of ECD and ECI increased progressively from the first instar to the last instar. The values of ECD varied from 03.98 – 11.25% and those of ECI from 03.85 – 10.80%. Thus there was an inverse relationship between the values of AD and those of ECD and ECI.
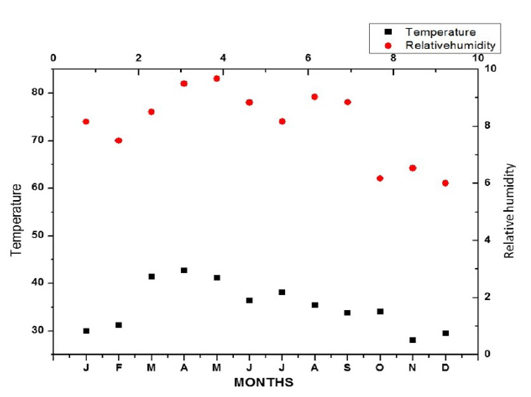
The total development time from egg laying to adult emergence was 15-22 days. The duration of life cycle may vary from our records depending on the prevailing temperatures and relative humidity (Figure 9). As no temperature margins occur study area at Kadapa, the duration of life cycle did not vary much over the overlapping seasons.
Over the entire period of its growth, a larva consumed on average over some of leaf material, with increased consumption in the last two instars, this tendency of greater consumption by the last two instars has been reported in lepidopterous larvae in general and it compensates for the energy expenditure of non-feeding pupal stage (Pandian, 1973). The values of CI are near to the range (05.95 – 11.54) predicted for forb foliage chewers (Slansky and Scriber, 1983). Food consumption rate depends on the conversion efficiency of ingested food to biomass (ECI), the rate increases as the conversion efficiency decreases and vice versa. In this sense, the high CI value (11.54) of instar I was probably due to low conversion efficiency and this character was reflected in the low values of ECI for instar I compared to other successive instars. Higher growth rates occur with penultimate than with final instars.
The standards of AD that were obtained in this study are comparable with the range of AD values (90 – 96.07%) for lepidopterous larvae (Pandian and Marian, 1986) .The average AD percentage is over 93 and this high AD substantiates the statement of Slansky and Scriber (1983), that foliage chewers often attain high AD values. Such high AD values also are expected when food item was rich in nitrogen (and also water). Similar results were repeated with Pieris brassicae (Yadava et al., 1979), Euploea core (Venkata et al., 2010), Ariadne merione merione (Atluri et al., 2010) and Junonia hierta (Harinath et al., 2014).
The values of ECD increase from early to last instar (Harinath et al., 2014). Such trend was observed with the ECDs of Lampides boeticus, with the lowest value in instar I and the highest in instar V. The ECDs obtained are low compared to the ADs and such low values are not unusual. This was indicative of low efficiency of conversion of digested food to biomass. This poor utilization of food was often attributed to deficiency in some essential nutrient in food (Bailey et al., 1976) or a factor causing an increase in energy expenditure on metabolism (Muthukrishnan, 1990). The pattern of ECI values followed closely the pattern of ECD. The values (1.50 – 14.80) obtained are comparable with the range of values expected for forb foliage chewers (1 – 78%). The pattern of ECI followed that of AD as suggested by Waldbauer (1968).
The values of ECD and ECI, mostly those of the last two instars, are also relatively high, thus indicating tissue growth efficiency respectively and ecological growth efficiency, which enabled Lampides boeticus to thrive fruitfully in the present study environment.
The information on the oviposition, larval host and larval performance in terms of food consumption, growth and utilization, and the length of life cycle from egg to adult emergence of Lampides boeticus in the present study may be profitably utilized in the successful protection management of this butterfly species in parks, zoological gardens and butterfly houses well planted with blossoming plants and with patches of shades.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest related to the work.
ACKNOWLEDGEMENTS
The Corresponding author Dr. S.P. Venkata Ramana, Department of Zoology, Yogi Vemana University greatly acknowledge the University Grants Commission (UGC), New Delhi for financial support through a Major Research Project and also sincere thanks to Andhra Pradesh Forest Department for giving permission for periodical survey in the forest areas.
AUTHOR’S CONTRIBUTION
The first and second authors collected the Butterfly life cycle stages and conducted captive breeding experiments in the laboratory. The third & corresponding author prepared and formatted the manuscript.
REFERENCES