South Asian Journal of Life Sciences
Research Article
Effect of Exogenously Administered Methyl Farnesoate and 20-OH Ecdysone on Polypeptide Profile of Vitellogenin and Vitellin in the Freshwater Crab Travancoriana schirnerae
Aswani Ayanath, Sudha Devi Arath Raghavan*
Department of Zoology, Mary Matha Arts & Science College, Wayanad, Kerala 670 645, India.
Abstract | This study evaluated the effects of exogenously administered methyl farnesoate (MF) and 20-OH ecdysone (20E) on polypeptide profile of circulating vitellogenin (Vg) and ovarian vitellin (Vn) in the edible freshwater crab Travancoriana schirnerae by sodium dodecyl sulphate-polyacrylamide gel electrophoresis. The results indicated that the polypeptide profiles of yolk proteins in the hemolymph and ovary of control and experimental females varied with the stage of development of the ovary. Our observations also revealed that both MF and 20E administration was able to induce the production of Vg and Vn during the avitellogenic and previtellogenic phases manifested by the presence of Vg and Vn polypeptide subunits in the electropherograms of experimentals over the controls. Moreover, the electropherograms of MF and 20E crabs during the vitellogenic phase displayed more number of Vg and Vn subunits with increased staining intensity and thickness compared to the control crabs. It was concluded that though both MF and 20E were able to induce or enhance the production of Vg and Vn during the avitellogenic, previtellogenic and vitellogenic phases, the effects were far more pronounced in MF injected females than the 20E injected crabs.
Keywords: Hemolymph, Ovary, SDS-PAGE analysis, Vitellin, Vitellogenin
Editor | Muhammad Nauman Zahid, Quality Operations Laboratory, University of Veterinary and Animal Sciences, Lahore, Pakistan.
Received | June 22, 2020 Accepted | July 15, 2020; Published | August 10, 2020
*Correspondence | Sudha Devi Arath Raghavan, Department of Zoology, Mary Matha Arts & Science College, Wayanad, Kerala 670 645, India; Email: arsudhadevi@gmail.com
Citation | Ayanath A, Raghavan SDA (2020). Effect of exogenously administered methyl farnesoate and 20-oh ecdysone on polypeptide profile of vitellogenin and vitellin in the freshwater crab Travancoriana schirnerae S. Asian J. Life Sci. 8(2): 55-63.
DOI | http://dx.doi.org/10.17582/journal.sajls/2020/8.2.55.63
ISSN | 2311–0589
Copyright © 2020 Ayanath and Raghavan. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Growth and differentiation of the oocyte in crustaceans can be divided into three phases: avitellogenic, previtellogenic and vitellogenic. The latter phase was characterized by the synthesis of the yolk protein, vitellogenin (Vg) in the hepatopancreas, its subsequent release to the hemolymph and incorporation by the developing oocytes (see reviews by Charniaux-Cotton, 1985; Meusy and Payen, 1988). The yolk protein precursor, Vg, was further modified with the addition of carbohydrate and lipid moieties to form vitellin (Vn) in which form it was stored in the oocytes for nourishing the embryo (see review by Meusy and Payen, 1988; Pateraki and Stratakis, 1997). In crustaceans, the synthesis of yolk may be endogenous (within the ovary) or exogenous (in the hepatopancreas) or both (see reviews by Meusy, 1980 and Tsukimura, 2001; Avarre et al., 2003; Auttarat et al., 2006). Endogenous vitellogenesis encompassed the formation of proteinaceous and glycoproteinaceous yolk which appeared as yolk globules in the oocytes whereas exogenous vitellogenesis involved the synthesis of lipid yolk in the hepatopancreas and its sequestration by the ovary (see review by Meusy, 1980).
The process of vitellogenesis was controlled by both neuroendocrine and non-neuroendocrine factors (Charmantier et al., 1997; Van Herp and Soyez, 1997). Neuroendocrine factors include the neurohormones: the gonad stimulating hormone (GSH), also called the vitellogenesis stimulating hormone (VSH) originating from the brain and thoracic ganglion and the gonad inhibiting hormone (GIH), also called the vitellogenesis inhibiting hormone (VIH) derived from the X-organ-sinus gland (XO-SG) complex of the eyestalk. The non-neuroendocrine secretions include the terpenoid hormone methyl farnesoate (MF) released from the mandibular organ and the ecdysteroids secreted by the Y organ. Many authors suggested the presence of a Vg stimulating ovarian hormone (VSOH) in follicle cells surrounding the vitellogenic oocytes (Picaud and Souty, 1980; Zerbib and Meusy, 1983).
In addition to the important roles played in the regulation of moulting, larval development and morphogenesis (Abdu et al., 1998; Reddy et al., 2004), MF, the crustacean equivalent of the juvenile hormone III of insects, functioned as a gonadotropin. The gonadotropic effect of MF and farnesoic acid (precursor to MF) has been reported in a number of crustaceans (Rodriguez et al., 2002; Mak et al., 2005; Tiu et al., 2006; Balasubramanian et al., 2010; Reddy and Reddy, 2015; Hemalatha et al., 2016; Sudha Devi and Aswani, 2018; Muhd-Farouk et al., 2019). In vitro studies by Otsu (1963) reported that MF promoted the development of oocyte and Vg messenger in the hepatopancreas and ovary. Exogenously administered MF enhanced the Vg titer in the hemolymph of eyestalk ablated Libinia emarginata (Vogel and Borst, 1989), Penaeus indicus (Nagaraju et al., 2002) and Macrobrachium malcolmsonii (Nagaraju et al., 2003). Medesani et al. (2012) specified the direct effect of MF on synthesis of ovarian proteins in the crayfish Cherax quadricarinatus.
Besides the prime function of controlling moulting and growth, crustacean ecdysteroids played crucial roles in reproduction (Chang et al., 2001; See reviews by Subramoniam, 2000, 2017). Several authors described the ecdysteroid induced stimulation of vitellogenesis, i.e. synthesis, release and transport of Vg to the ovary (Kanazawa and Teshima, 1971; Chaix and De Reggi, 1982; Souty et al., 1982). In the amphipod Orchestia gammarella, high 20-OH ecdysterone (20E) concentration was vital for Vg production in the hepatopancreas (Blanchet-Tournier, 1982). Steel and Vafopoulou (1998) stated that the hemolymph Vg titer paralleled ecdysteroid titer during vitellogenesis in isopods and amphipods. Protein synthesis in the ovary and integuementary tissues was heightened following 20E administration in the sand crab Emerita asiatica (Gunamalai et al., 2004). Tiu et al. (2006) described the relationship between the hemolymph ecdysteroid titer and Vg gene expression in the ovary and hepatopancreas of P. monodon.
Travancoriana schirnerae is an edible freshwater crab commonly distributed in the wetlands of Wayanad, Kerala. The ovary consumed by the native tribes during the breeding season, is a very good source of inexpensive, high quality protein. Though ample literature is available regarding the role of MF and ecdysteroids on yolk protein synthesis in marine decapods, literature is meager on freshwater brachyurans and a detailed study in this regard is required to establish this. The current study evaluated the effects of exogenously administered MF and 20E on levels of circulating Vg and ovarian Vn profile during different stages of development of the ovary in the freshwater crab T. schirnerae, which is an important step in understanding the process of vitellogenesis of the species under study.
MATERIALS AND METHODS
Adult intermoult crabs (CW: 3.5-4.5 cm; weight: 35-40 g) in various oogenic stages (n=45) were collected over a period of one year from January 2019 to December 2019 from the paddy fields of Ondayangadi, Wayanad, Kerala (11°49ˈ20.3̎ N and 76°01ˈ47.1̎ E). The specimens were transported to the laboratory alive, kept in well-aerated circular plastic basins (diameter 46 cm, depth 20 cm) (5 individuals per basin) containing well water for acclimatization. The crabs were fed ad libitum with boiled egg, pieces of cooked beef liver and decaying aquatic vegetation, daily once during the acclimatization (2-3 days) and experimental period. The water in the basins were changed daily, one hour after feeding. The temperature and photoperiod conditions were maintained (25±3°C and 12L: 12D) through the experimental period.
Experimental Design
The individuals were distributed into three groups (n=15). The carapace width (body size) and body weights (Shimadzu electronic top loading balance, sensitivity 0.001 g) were recorded for all the specimens. Group I comprised of intact crabs without treatment while Groups II and III were given injections of MF (trans, trans MF; Echelon Biosciences, Salt Lake City, USA) and 20E (Sigma Chemicals, USA) at doses of 25 and 40 ng/g body weights, respectively. The injections were given weekly once, on the first, seventh, fourteenth and twenty first day of the experiment through the arthrodial membrane of the coxal segment of the third walking leg. Both controls and experimentals were sacrificed one week after the last injection (on day 28). For analysis of the yolk proteins, the hemolymph and ovarian samples collected from the control and experimental groups were subjected to sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE).
Preparation of Hemolymph Sample for Sds-Page Analysis
The hemolymph samples (approximately 500 µL each) collected separately from females in various oogenic stages (avitellogenic, early previtellogenic, late previtellogenic, early, middle and late vitellogenic stages (n=5 each) by puncturing the walking legs were placed in sterile centrifuge tubes. The samples in each oogenic stage were pooled and 100 µL of the sample was mixed with Trichloroaceticacid (10%) and centrifuged (REMI, India) at 2000 rpm for 10 min. The pellets were collected and washed in distilled water and homogenized in SDS sample buffer containing 10% SDS, glycerol, β-mercapto-ethanol, 0.1% bromophenol blue and 0.5 M Tris-HCl (pH 6.8), using an ice-cold homogenizer. The homogenate was denatured in a boiling water bath for 5 minutes and then cooled immediately. The supernatant obtained after centrifugation at 2000 rpm for 30 min was used for SDS-PAGE analysis.
Preparation of Ovarian Homogenates
Pieces of ovary from various oogenic stages (n=5; 10 mg each) were homogenized in extraction buffer containing 10% SDS, glycerol, β-mercapto-ethanol, 0.1% bromophenol blue and 0.5 M Tris-HCl (pH 6.8) with a mortar and pestle. The homogenates were denatured in boiling water bath for 5 minutes and cooled immediately. The samples were centrifuged at 2000 rpm for 30 minutes. After centrifugation, a lipid cap was formed at the top of the centrifuge tube and pellets of cell debris deposited at the bottom. The aqueous supernatant was carefully transferred with a micropipette to another fresh tube and used as sample for SDS-PAGE analysis.
Electrophoresis
The SDS-PAGE was performed according to the method of Laemmli (1970) using a 7% gradient slab gel. The samples and standard (10μL each) were loaded on a 4% stacking gel. The separation of polypeptide fractions was conducted with an electrophoresis buffer containing 25mM Tris, 200mM glycine and 0.1% SDS, pH 8.3, in a vertical slab gel electrophoresis unit (16x14cm gel, Biotech, India). The initial voltage was maintained at 60V till the tracking dye, bromophenol blue, touched the lowermost portion of the stacking gel and then increased to 100V. The electrophoretic run was continued for 5 hours, until the dye front reached the bottom line of the resolving gel.
Once the electrophoretic run completed, the gels were transferred to 50% methanol in order to fix the polypeptide bands and stained with 0.1% Coomassie Brilliant Blue R-250 (50% distilled water, 40% methanol and 10% acetic acid mixture) for 1 hour at room temperature. The gel was then transferred to the destaining solution (distilled water, methanol and acetic acid mixture in 50:40:10 ratio) in which it was stored. After destaining, the gels were photographed using a Sony HX 400V digital camera. The molecular weights of polypeptide subunits were determined by comparing their relative mobility in SDS-PAGE with those of the standard protein markers (New England BioLabs, UK) of known molecular weights.
RESULTS
Travancoriana schirnerae was an annual breeder, accommodating a single ovarian cycle. The annual ovarian cycle was divided into 3 phases: avitellogenic (April-May), previtellogenic (June-September) and vitellogenic (October-March). The vitellogenic phase was further divided into early (October), middle (November-January) and late (February-March) stages. Our observations revealed that the electrophoretic profiles of yolk proteins in the hemolymph and ovary of control and experimental females varied with the stage of development of the ovary (Figures 1-2).
Effect of Mf and 20E Administration on Yolk Protein Concentration in Avitellogenic and Previtellogenic Phases
The electropherograms of the hemolymph and ovarian homogenates of control crabs during avitellogenic phase did not display any polypeptide subunits corresponding to the yolk proteins: Vg and Vn. On the other hand, SDS-PAGE analysis of the hemolymph and ovary of MF injected crabs revealed two Vg subunits with molecular weights 105.96 and 148.31 kDa and two Vn subunits with molecular weights 97.96 and 141.56 kDa. Of the Vg subunits, the 148.31 kDa band stained intensely and appeared as the major subunit and the 105.96 kDa band which stained mildly with Coomassie Blue was identified as the minor band. The heavily stained 141.56 kDa Vn band was considered as the major band and the faintly stained 97.96 kDa as the minor band. On the other hand, only one subunit each was detected for Vg (148.31 kDa as major band) and Vn (112.91 kDa as minor band) in the electropherograms of 20E injected crabs (Figure 1A).
No polypeptide subunits corresponding to the yolk proteins were perceived in the electropherograms of control crabs in previtellogenic phase. Two moderately stained subunits representing Vg (148.31 as major band and 105.96 kDa as minor band) and two representing Vn (97.96 as minor and 141.56 kDa as major band) were apparent in the hemolymph of MF treated crabs during the early previtellogenic phase (June-July). Both the Vg subunits appeared deeply stained by late previtellogenic phase (August-September). The electrophoretic profile of MF injected ovary during late previtellogenic phase has shown three Vn subunits (the 97.96 kDa as minor band and 112.91 and 141.56 kDa as major bands) (Figures 1B-C).
Vitellogenin protein extracted from hemolymph of 20E injected crabs during the early previtellogenic phase was resolved into two moderately stained polypeptide subunits with molecular weights 105.96 (minor band) and 148.31 kDa (major band). Both the subunits became intensely stained major bands in the late previtellogenic phase. The two Vn subunits presented in the ovarian extracts of 20E injected crabs during early previtellogenic phase were the 97.96 (minor band) and the 141.56 kDa subunits (major band) whereas the polypeptide fractions noticed in the late previtellogenic phase were the 112.91 and 141.56 kDa bands, of which the latter was lightly stained and the former heavily stained during August. Both the bands were perceptible as intensely stained major bands during September (Figures 1B-C).
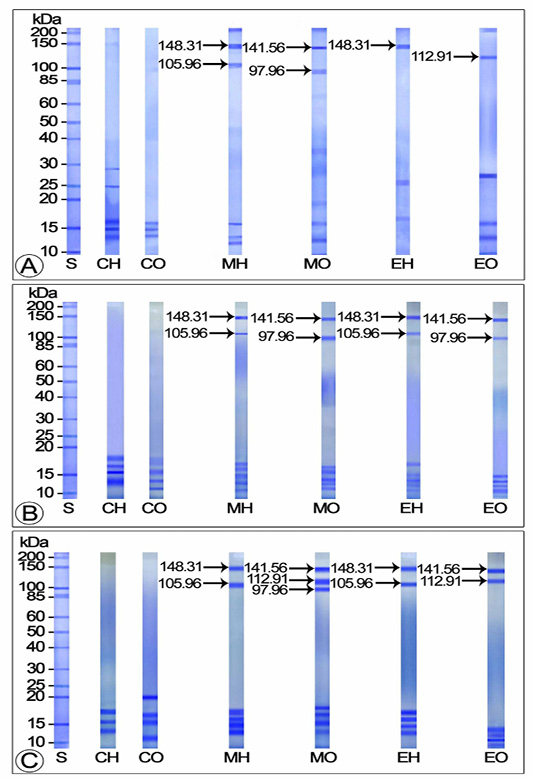
Figure 1: Electropherogram of yolk proteins in hemolymph and ovary of control and experimentals during avitellogenic and previtellogenic phases (A) Avitellogenic phase (April) (B) Early previtellogenic phase (June) (C) Late previtellogenic phase (September) CH: Hemolymph of control crabs; CO: Ovary of control crabs; EH: Hemolymph of 20E injected crabs; EO: Ovary of 20E injected crabs; MH: Hemolymph of MF injected crabs; MO: Ovary of 20E injected crabs; S: Standard marker protein
Effect of Mf and 20E Administration on Yolk Protein Production during Vitellogenic Phase
The hemolymph samples of control and 20E injected crabs exhibited three Vg subunits each (105.96, 148.31 and 169.37 kDa) during the early vitelogenic stage. In both control and 20E injected crabs, these subunits were observed as deeply stained major bands, however, in 20E injected crabs, the 148.31 kDa subunit seemed to be thicker than the other two (105.96 and 169.37 kDa). Compared to the control and 20E injected groups, four Vg subunits were identified in MF injected crabs (97.96, 105.96, 148.31 and 169.37 kDa), of which the three intensely stained (except the 97.96 kDa minor band) bands were considered as the major subunits. Two Vn subunits each (112.91 and 141.56 kDa) were displayed in the electropherograms of ovarian extracts from control and 20E injected crabs during this stage. In 20E treated crabs, these bands were detected as heavily stained major bands whereas in controls, they remained as mildly stained minor bands. Three intensely stained polypeptide fractions with apparent molecular masses 97.96, 112.91 and 141.56 kDa were noticed in the ovarian extracts of MF injected groups, of which the 97.96 (minor band) and 141.56 kDa (major band) subunits stained intensely while the 112.91 kDa subunit (major band) stained faintly (Figure 2A).
On SDS-PAGE, the hemolymph extract of control, 20E and MF injected crabs in middle vitellogenic stage presented three Vg polypeptide subunits each having molecular masses 105.96, 148.31 and 169.37 kDa. In control crabs, all the subunits were spotted as moderately stained thin bands while they appeared thick and deeply stained in MF and 20E injected females. Four Vn subunits each (97.96, 112.91 and 141.56 and 200 kDa) were released from the ovarian extracts of control and 20E injected crabs during this stage. All the subunits in controls and 20E treated crabs stained intensely, nevertheless, the 112.91 and 141.56 kDa bands of 20E injected crabs appeared thicker than those of the controls. Electrophoretic analysis of ovary from MF injected crabs demonstrated five heavily stained Vn bands (71.41, 97.96, 112.91 and 141.56 and 200 kDa), of which three (97.96, 112.91 and 141.56 kDa) appeared as main bands and the remaining two (71.41 and 200 kDa) as minor bands (Figure 2B).
The electrophoretic profile of the hemolymph and ovarian extracts of controls and experimentals during the late vitellogenic stage unveiled three Vg polypeptide fractions (105.96, 148.31 and 169.37 kDa) which were moderately stained in control and 20E injected groups and intensely stained in MF injected group. Of the four deeply stained Vn subunits (71.41, 97.96, 112.91 and 141.56 kDa) apparent in the ovary of control and 20E injected crabs, the 112.91 and 141.56 kDa subunits observed as major bands and the 71.41 and 97.96 kDa subunits as minor bands. On the other hand, the Vn protein of MF injected crabs was composed of heavily stained five subunits with molecular masses 71.41, 97.96, 112.91, 141.56 and 181.71 kDa; among which, three subunits (71.41, 112.91 and 141.56 kDa) appeared as main bands and the remaining two (97.96 and 181.71 kDa) were minor bands (Figure 2C).
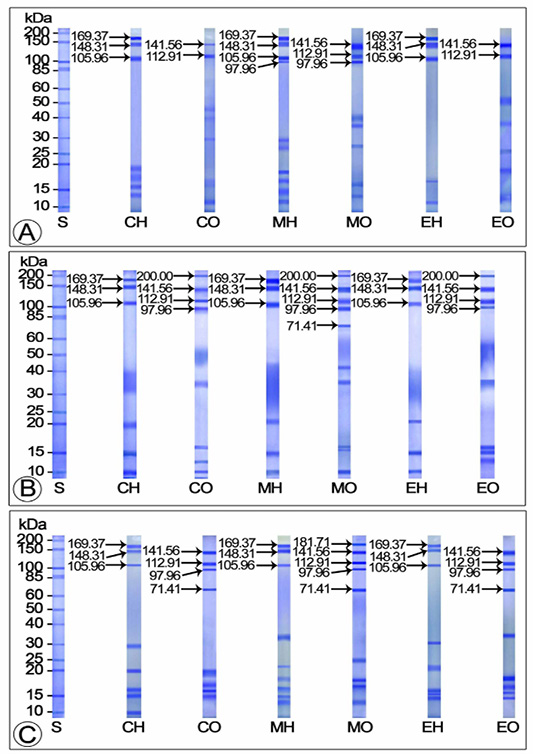
Figure 2: SDS-PAGE separation of yolk proteins of hemolymph and ovary of control and experimentals during vitellogenic phase (A) Early vitellogenic stage (October) (B) Middle vitellogenic stage (January) (C) Tertiary vitellogenic stage (March) CH: Hemolymph of control crabs; CO: Ovary of control crabs; EH: Hemolymph of 20E injected crabs; EO: Ovary of 20E injected crabs; MH: Hemolymph of MF injected crabs; MO: Ovary of 20E injected crabs; S: Standard protein marker
DISCUSSION
This study evaluated the effects of exogenous administration of MF and 20E on yolk protein profiles in the hemolymph and ovary during various stages of the ovarian cycle in the edible freshwater crab T. schirnerae. Our observations revealed that both MF and 20E administration not only induced the production of yolk proteins during the avitellogenic and previtellogenic phases but also increased their production during the vitellogenic phase.
The current study confirmed the presence of four Vg polypeptide subunits having molecular masses 97.96, 105.96, 148.31 and 169.37 kDa in the hemolymph of T. schirnerae. In crustaceans, the number and molecular masses of subunits constituting the yolk protein Vg varied with species (Tsukimura, 2001; Auttarat et al., 2006). For example, the Vg of the Chinese mitten crab Eriocheir sinensis (Chen et al., 2004) and blue crab Callinectes sapidus (Zmora et al., 2007) was made up of two subunits with dissimilar masses. Three polypeptide subunits were identified in the Vg of Potamon potamios (Pateraki and Stratakis, 1997), P. pelagicus (Ravi and Manisseri, 2011) and the freshwater prawn M. malcolmsonii (Shanju and Geraldine, 2010). Zacharia and Kakati (2004) described the occurrence of six Vg subunits in P. merguiensis.
In crustaceans, the number of Vn subunits in the ovary varied from 2-8 (Quinitio et al., 1990; Vafopoulou and Steel, 1995). The electrophoretic analysis of the ovary of control and experimental T. schirnerae revealed six Vn polypeptide subunits with molecular weights 71.41, 97.96, 112.91, 141.56, 181.71 and 200 kDa. In contrast to our observations, two fractions were reported in Charybdis feriatus (Komatsu and Ando, 1992a), E. japonicus (Komatsu and Ando, 1992a) and M. malcolmsonii (Shanju and Geraldine, 2010); three in Sicyonia ingentis (Tsukimura et al., 2000); four in M. rosenbergii (Komatsu and Ando, 1992a), C. sapidus (Lee and Watson, 1995) and P. potamios (Pateraki and Stratakis, 2000), five in Procambarus clarkii (Lui and O’Connor, 1976) and P. chinensis (Chang et al., 1996) and eight in P. monodon (Chang et al., 1993). From the above observations including the current one, it is concluded that species specific differences occur in the number and molecular weights of polypeptide subunits constituting the yolk protein Vn.
From the results, it was clear that in T. schirnerae, vitellogenic stage specific variations occur in the number and molecular masses of subunits constituting Vg and Vn. Comparable situations were reported in P. semisulcatus, where the Vg encompassed two subunits with molecular masses 74 and 199 kDa and Vn consisted of four subunits with molecular masses 72, 79, 100 and 207 kDa (Avarre et al., 2003). In C. sapidus, two subunits with molecular weights 78.5 and 207.3 kDa (Zmora et al., 2007) were identified in the hemolymph and four with molecular weights 86, 109, 168 and 188 kDa in the ovary (Lee and Watson, 1995). In contrast, identical polypeptide fractions were apparent in the Vg (89, 100 and 170 kDa) and Vn (89 and 100 kDa) of M. malcolmsonii (Shanju and Geraldine, 2010). A similar trend was observed in the hemolymph and ovary of M. rosenbergii (90, 102 and 199 kDa) (Wilder et al., 1994) and P. potamios (85, 105 and 115 kDa) (Pateraki and Stratakis, 1997; 2000).
Our observations revealed that MF administration induced the production of yolk proteins during the avitellogenic and previtellogenic phases as demonstrated by the presence of Vg and Vn bands in the electropherograms of the experimentals over the controls. Similar results were obtained in Oziothelphusa senex senex, wherein significant increase in ovarian Vg level was discerned with MF injection during the immature stage (Reddy and Reddy, 2015). The ovarian Vg level was significantly high in M. rosenbergii treated with MF during the previtellogenic phase (Hemalatha et al., 2016). In T. schirnerae, the electropherograms of MF injected crabs during the vitellogenic phase exhibited more number of Vg and Vn subunits with increased staining intensity and thickness compared to the 20E injected and control crabs. In agreement with our findings, in P. clarkii, MF administration induced the production of yolk proteins in the ovary during vitellogenesis (Rodriguez et al., 2002). Studies of Mak et al. (2005) with farnesoic acid in the red crab C. feriatus have shown that Vg gene expression was negligible in the non-reproductive phase, reached its maximum through middle vitellogenesis and dropped to minimum in late vitellogenesis. In C. quadricarinatus, it was reported that significant accumulation of Vg was perceptible in ovaries treated with MF during early pre-reproductive period and not during the reproductive period (Medesani et al., 2012). In contrast, in Neohelice granulata, MF administration boosted the Vg and Vn levels in the hemolymph and ovary only during the post-reproductive period (Medesani et al., 2015).
From the above observations including the present one, it is very clear that MF has an important role in the synthesis, release and transportation of Vg into the ovary. One possible explanation for the appearance of Vg and Vn proteins in the hemolymph and ovary of avitellogenic and previtellogenic ovaries is that the exogenously administered MF might have triggered the production of Vg in the hepatopancreas, its discharge to the hemolymph and storage by the ovary. The reason for the manifestation of increased number of Vg and Vn subunits with increased staining intensity and thickness in MF injected females compared to the 20E injected and control crabs during the vitellogenic phase is that the exogenously administered MF enhanced Vg uptake and its conversion to Vn by the ovary during the vitellogenic phase.
However, there were occasions when MF administration had no effect on Vg or Vn production. For instance, no positive correlation was found between the hemolymph Vg titer and MF administration in senescent females of Homarus americanus (Tsukimura et al., 1993) and Triops longicaudatus (Riley and Tsukimura, 1998) and S. ingentis (Balasubramanian et al., 2010).
In T. schirnerae, 20E treatment induced the production of Vg and Vn during the avitellogenic and previtellogenic phases as evinced from the presence of Vg and Vn bands in the electropherograms of experimentals over the controls. In support of this, in the same species, the same authors proved histologically that 20E injection induced the avitellogenic and previtellogenic ovaries to grow into vitellogenic ovaries (Sudha Devi and Aswani, 2019). In P. vannamei, Chan (1995) witnessed the accumulation of ovarian polypeptides with increase in ovarian ecdysteroid levels during secondary vitellogenesis. The parallelism between hemolymph Vg concentration and ecdysteroid titer during vitellogenesis was reported in isopods, amphipods and crabs, signifying their role in Vg synthesis (Shih, 1997; Steel and Vafopoulou, 1998; Zapata et al., 2003). Mc Carthy (1980) and Kanazawa and Teshima (1971) described the possible involvement of ecdysteroids in exhilarating Vg synthesis and release into the hemolymph and or its uptake by the ovary.
Conversely, in vitro administration of 20E did not induce Vg synthesis in stage I ovary of P. vannamei (Chan, 1995). In Carcinus maenas, the process of vitellogenesis was continued even after Y-organectomy (Demeusy, 1962). In M. rosenbergii, Okumura and Aida (2000) did not find any involvement of ecdysteroids in ovarian development.
The mode of operation of MF and 20E in the control of Vg and Vn production is still unclear. MF may act directly by triggering the production of Vg in the hepatopancreas or augments the release of Vg into the hemolymph and incorporation by the ovary or indirectly via the VSH that promotes the hepatopancreas to synthesize and secrete Vg into the hemolymph and its assimilation by the ovary. In decapod crustaceans ecdysteroids may act directly via receptor (EcR) facilitated transport of Vg into the ovary with ecdysteroids in their oocytes (Warrier and Subramoniam, 2002) or indirectly via the VSOH for the synthesis of Vg in the hepatopancreas and its uptake by the ovary (Kanazawa and Teshima, 1971, Yano and Chinzei, 1987). The presence of ecdysteroids possibly stimulate the ecdysone responsive element in the upstream of the Vg gene which in turn initiate the transcription of Vg gene (Tokishita et al., 2006). In O. senex senex, Girish et al. (2015) described increased EcR expression in the hepatopancreas and activation of ecdysteroid responsive gene during vitellogenesis stage I and II.
To conclude, this study proved that exogenous administration of MF and 20E in T. schirnerae induced the production of Vg and Vn during the avitellogenic and previtellogenic phases. The electropherograms of MF and 20E injected crabs during the vitellogenic phase displayed more number of Vg and Vn bands with increased staining intensity and thickness compared to the control crabs. Though both MF and 20E were able to stimulate the production of Vg and Vn during the avitellogenic and previtellogenic phases, the effects were far more pronounced in MF injected crabs than 20E injected ones. Further molecular studies pertaining to the receptor activities of Vg and Vn are needed to solve this problem.
ACKNOWLEDGEMENTS
The financial support provided by the Kerala State Council for Science Technology & Environment (Order No. P 115/2016/KSCSTE, dated 03-05-2016) in carrying out this research is gratefully acknowledged.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest related to the work.
authors contribution
All authors contributed eqaully.
REFERENCES





