South Asian Journal of Life Sciences
Research Article
Biological Evaluation of New Chemistry and IGR Insecticides Against Spodoptera litura F.
Rashid Ahmed Khan*, Muhammed Naveed
Plant Protection Division (PPD), Nuclear Institute for Agriculture & Biology (NIAB), Jhang Road, Faisalabad, Pakistan
Abstract | The present study was designed to study the lethal effects of new chemistry, conventional and IGRs insecticides using a diet incorporation bioassay against second and third instar S. litura. Initially all the insecticides were used in recommended field doses for their lethal effects and later on all the IGRs and new chemistry insecticides were evaluated for their lethal effects keeping in view their environmental safety. The results showed among insecticides Emamectin Benzoate, a bacterial metabolite, proved highly effective and caused 100 percent mortality of the insects followed by Chlorpyriphos, a conventional organophosphate (OP) insecticide. Among IGRs Lufenuron was the most effective insecticide by causing 100 percent mortality in the insects exposed. These findings will help us to devise bio-rational IPM strategies against S. litura in agro-ecosystem.
Keywords: Lethal effects; New chemistry insecticides; IGRs; S. litura; Bio-rational IPM
Editor | Muhammad Nauman Zahid, Quality Operations Laboratory, University of Veterinary and Animal Sciences, Lahore, Pakistan.
Received | December 29, 2019 Accepted | May 23, 2020; Published | August 10, 2020
*Correspondence | Rashid Ahmed Khan, Plant Protection Division (PPD), Nuclear Institute for Agriculture & Biology (NIAB), Jhang Road, Faisalabad, Pakistan; Email: rashidpp2004@yahoo.co.uk
Citation | Khan RA, Naveed M (2020). Biological evaluation of new chemistry and iGR insecticides against spodoptera litura f. S. Asian J. Life Sci. 8(2): 49-54.
DOI | http://dx.doi.org/10.17582/journal.sajls/2020/8.2.49.54
ISSN | 2311–0589
Copyright © 2020 Khan and Naveed. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Cotton leafworm, Spodoptera litura is considered one of the most destructive pest in Asian tropics and widely distributed throughout temperate and tropical Asia, Pacific islands and Australia (Feakin, 1973; Kranz et al., 1977). It is a polyphagous pest with the great potential to spread and invade new crop areas and may adapt in new ecological environments (Brown and Dewhurst, 1975; Holloway, 1989).
S. litura lays eggs in clusters on the leaf upper surface. Temperature of 37 ºC is considered upper developmental threshold and 40°C is lethal temperature (Rao et al., 1989). The flight range of moths is about 1.5 Km which helps in dispersion and oviposition on different hosts (Salama and Shoukry, 1972).
S. litura infest a wide variety of field crops with profound economic impact. It has been reported to cause damages to tomatoes, potatoes, sugarbeet (Patnaik, 1998; Trivedi, 1988; Chatterjee and Nayak, 1987; Singh and Sethi, 1993). In Pakistan it also attack a wide range of crops namely cotton and rice (Ahmad and Kamaluddin, 1987) besides it damages cabbage, turnip, spinach, tomato, cauliflower, carrot, onion, bringal (Maree et al., 1999).
In recent years S. litura has developed resistance to insecticides belonging to different groups (Ramakrishnan et al., 1984; Abbas et al., 2014). The control of this pest is therefore a serious problem. Newer insecticides are being tested with promising results (Venkateswarlu et al., 2005). New chemistry insecticides such as spinosad, emamectin benzoate and chlorantraniliprole have been proved very effective against S. litura (Gadhiya et al., 2014; Patil et al., 2014). Other insecticides such as flubendiamide and chlorfenapyr also proved very effective on different vegetable crops (Chatterjee and Mondal, 2012). Sublethal effects of mathoxyfenozide was evaluated on changes in population dynamics and sterility of S. litura and found mathoxyfenozide a potent compound for the management of this pest (Shahout et al., 2011). Along with conventional insecticides bioinsecticides have also been evaluated as part of the organic pest management. Plant oil mixture with synthetic pyrethroid mixture gave higher mortality compared to the synthetic pyrethroid only (Suganthy and Sakthivel, 2013; Anju and Srivastava, 2012).
The current investigations were carried out to study the lethal effects of different classes of insecticides such as, biological based, new chemistry, conventional and IGR insecticides against S. litura under laboratory conditions. Output of this investigation will help to screen the most effective insecticides as well as environmentally safe insecticides which may be incorporated into biorational IPM program.
Material and Methods
Maintenance of Insect Colony
The susceptible population of S. litura was reared on semi artificial diet in an insectary for fifteen generations without exposure to any insecticide on temperature, humidity and photoperiod maintained at 25± 2 °C, 45 5 and 12L: 12D. Freshly hatched neonates were carefully transferred collectively with the help of camel hair brush to the artificial diet. Freshly emerged adults were transferred to glass container with cotton cloth in the base and on the top with a vial containing 10% honey solution.
Preparation of Artificial Diet
Artificial diet for rearing and bioassay was prepared in the laboratory, containing well homogenized ingredients such as, chickpea powder 100 g, agar 12.8 g, sorbic acid 1 g, yeast 30 g, choline chloride 10% 7.20 ml, vitamin (ABCDE) 2 ml, ascorbic acid 3.20 g, streptomycin sulphate 0.04 g and a total of 800 ml of distilled water.
Insecticide Treatment
A total of ten insecticides belonging to different insecticides viz a viz., Lambda Cyhalothrin, Emamectin Benzoate, Fipronil, Methoxyfenozoide, Pyriproxyfen, Buprofezin, Spinosad, Chlorpyriphos, Thiamethoxam and Lufenuron were used in recommended doses in distilled water (Table 1).
Diet Incorporation Insect Bioassay
All the insecticides were prepared in recommended field doses or in ppm in distilled water before incorporation into the artificial diet. Recommended doses were introduced into the artificial diet and homogenized and kept on room temperature for 8 h to allow evaporation of acetone alone was used as a control. The treated artificial diet weighing 2 g each was dispensed into 10 ml capacity transparent cups with aeration net in the lid. A total of 20 cups per concentration were prepared for each treatment and each cup was introduced with 5 second instar larvae. The mortalities among insects were observed 24, 48 and 72 h post treatment. The mortality data were analyzed by POLO-Plus (Finney, 1971).
Data Analysis of Insect Mortality
The mortality date were analyzed by the method [20] using a computer application, POLO Plus. Significance of the LC50 values were confirmed if their 95% Fiducial Limits (FLs) did not overlap (Litchfield and Wilcoxon, 1949). Toxicity Ratios (TR) of insecticides was determined using the formula:
Toxicity Ratio (TR) = LC50 value of the most effective insecticide / LC50 value of candidate insecticide (Jotwani et al.,1971; Lan and Zaho 2003).
Results
Efficacy of all the ten insecticides used in recommended field doses for the evaluation against S. litura varied significantly after 72 h post treatment (Table 2). Emamectin benzoate, with 100 percent mean percent mortality was the most effective insecticide followed by Lufenuron against second instar larvae. Other insecticides were ranked with regard to mean percent mortality as, Pyriproxyfen> Fipronil> Chlorpyriphos> Methoxyfenozoide> Spinosad> Buprofezin. Lambda Cyhalothrin and Thiamethoxam did not cause any mortality and were statistically non-significant and placed in one group. The relative toxicity of Emmamectin benzoate and Lufenuron remained at 1.
Almost similar trend in mean percent mortalities was observed for third instar larvae of S. litura. Slight reduction in mortalities was observed for third instar larvae (Table 3). Highest efficacy was recorded for Pyriproxyfen followed by Emamectin Benzoate. Other insecticides were ranked according to their mean percent mortality as Lufenuron> Chlorpyriphos> Fipronil> Methoxyfenozoide. Lambda Cyhalothrin, Buprofezin, Spinosad and Thiamethoxam did not cause any mortality and remained statistically non-significant among one another. The relative toxicity for only Pyriproxyfen remained at 1. The resistance ratio of third instar over second instar did not change and remained at 1.
The IGRs such as Pyriproxyfen, Lufenuron, Methoxyfenozoide and a new chemistry insecticide, Fipronil were further evaluated in serial dilution for more accurate estimate. Probit analysis of the data showed that Fipronil was the most effective IGR with LD50 value of 0.73 ppm followed by Lufenuron, Pyriproxyfen and Methoxyfenozoide with LD50 values of 1.20, 15.09 and 20.46 ppm, respectively against second instar larvae of S. litura, 24 h post treatment. The LD50 values proportionately reduced with the increase in mortality values (Table 4). The mortality value of Fipronil reduced to 0.46 and 0.36 ppm after 48 and 72
Table 1: Details of insecticides for the evaluation against S. litura under laboratory conditions
| Sr. | Trade name | Active ingredient (AI) | Formulation | Manufacturer | Dose range |
| 1 | Karate® | Lambda Cyhalothrin | 2.5 EC | Syngenta | 330ml |
| 2 | Proclaim® | Emamectin Benzoate | 19 EC | Syngenta | 200ml |
| 3 | Regent® | Fipronil | 80 WG | Syngenta | 30gm |
| 4 | Runner® | Methoxyfenozoide | 240 SC | Arysta LifeSciences | 200-400ml |
| 5 | Hy-flow® | Pyriproxyfen | 10.5 EC | M/S Jiangsu Kuaida Agrochemicals Co., Ltd, China | 250-500ml |
| 6 | Buprofezin® | Buprofezin | 25 WP | Welcare Chemicals corporation | 500gm |
| 7 | Tracer® | Spinosad | 240 SC | Arysta LifeSciences | 40-80ml |
| 8 | Lorsban® | Chlorpyriphos | 40 EC | Bayer | 250ml |
| 9 | Actara® | Thiamethoxam | 25WP | Syngenta | 24gm |
| 10 | Lufenuron® | Lefnuron | 5 EC | Jiangsu flag chemical industry Co., Ltd, China |
200ml |
Table 2: Effect of different insecticides on second instar larvae of S. litura at different interval post treatment under laboratory conditions.
| Insecticides | Insects (n) | Mean Percentage Mortality (24 h) | Mean Percentage Mortality (48 h) | Mean Percentage Mortality (72 h) |
| Lambda Cyhalothrin | 90 |
0E |
0D |
0C |
| Emamectin Benzoate | 90 |
100A |
100A |
100A |
| Fipronil | 90 |
20 D |
93.33AB |
96.667A |
| Methoxyfenozoide | 90 |
6.66DE |
66.66C |
76.66B |
| Pyriproxyfen | 90 |
93.3AB |
96.66AB |
96.66A |
| Buprofezin | 90 |
0E |
3.33D |
3.33C |
| Spinosad | 90 |
0E |
0D |
10C |
| Chlorpyriphos | 90 |
80BC |
90B |
93.33A |
| Thiamethoxam | 90 |
0E |
0D |
0C |
| Lufenuron | 90 |
66.66C |
100 A |
100A |
| LSD Critical Value for Comparison | 17.86 | 8.22 |
15.85 |
|
Any two means in the same column followed by the same letter are statistically not significantly different at P = 0.05; LSD.
Table 3: Effect of different insecticides on third instar larvae of S. litura at different interval post treatment under laboratory conditions.
| Insecticides | Insects (n) | Mean Percentage Mortality (24 h) | Mean Percentage Mortality (48 h) | Mean Percentage Mortality (72 h) |
| Lambda Cyhalothrin | 90 |
0B |
0D |
0D |
| Emamectin Benzoate | 90 |
76.66A |
83.3AB |
93.3A |
| Fipronil | 90 |
3.3B |
33.3C |
76.6BC |
| Methoxyfenozoide | 90 |
0B |
46.6C |
70C |
| Pyriproxyfen | 90 |
83.3A |
86.6A |
96.6A |
| Buprofezin | 90 |
0B |
0D |
0D |
| Spinosad | 90 |
0B |
0D |
0D |
| Chlorpyriphos | 90 |
10B |
66.6B |
83.3B |
| Thiamethoxam | 90 |
0B |
0D |
0D |
| Lufenuron | 90 |
0B |
93.3A |
93.3A |
| LSD Critical Value for Comparison | 13.19 | 18.65 |
8.79 |
|
Table 4: Toxicity of selected IGRs and a new chemistry insecticide against second instar larvae of S. litura at 24, 48 and 72 h under laboratory conditions
| Insect Growth Regulator (IGRs) | Time (h) |
Insects (n) |
LC 50 (ppm) |
LC90 (ppm) |
Slope ±SE |
χ2 |
*TR |
| Pyriproxyfen | 24 | 250 | 15.09 (11.24-20.45) | 86 | 1.69 (0.39) | 0.26 | 0.04 |
| 48 | 250 | 7.92 (4.67-14.15) | 84.01 | 1.25 (0.20) | 3.03 | 0.05 | |
| 72 | 250 | 1.20 (0.79-16.94) | 11.17 | 1.32 (0.23) | 0.66 | 0.20 | |
| Lufenuron | 24 | 250 | 3.79 (7.03-1152) | 6.35 | 2.26 (0.34) | 7.88 | 0.19 |
| 48 | 250 | 1.72 (0.14-6.59) | 5.42 | 1.79 (0.38) | 1.74 | 0.26 | |
| 72 | 250 | 1.04 (0.22-2.72) | 1.44 | 0.81 (0.46) | 1.27 | 0.34 | |
| Methoxyfenozoide | 24 | 250 | 20.46 (11.01-41.33) | 183.14 | 1.34 (0.23) | 1.96 | 0.03 |
| 48 | 250 | 9.21 (7.20-11.92) | 68.91 | 1.46 (0.21) | 0.80 | 0.04 | |
| 72 | 250 | 5.92 (2.34-14.83) | 59.25 | 1.28 (0.26) | 5.79 | 0.06 | |
| Fipronil | 24 | 250 | 0.73 (0.15-2.0) | 6.37 | 1.36 (0.33) | 0.91 | 1 |
| 48 | 250 | 0.46 (0.17-0.99) | 4.87 | 1.25 (0.35) | 0.25 | 1 | |
| 72 | 250 | 0.36 (0.78-1.22) | 2.66 | 1.47 (0.74) | 1.12 |
1 |
*TR = Toxicity Ratio is the LC50 of standard insecticide divided by LC50 of candidate insecticide
h post treatment. The toxicity value of only Fipronil remained at 1.
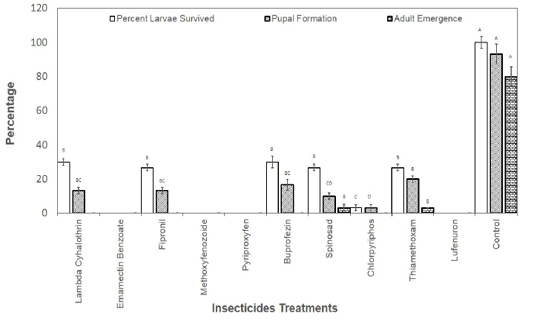
Figure 1: Percentage of second instar larvae those survived the effect of insecticides and emerged as adult insects.
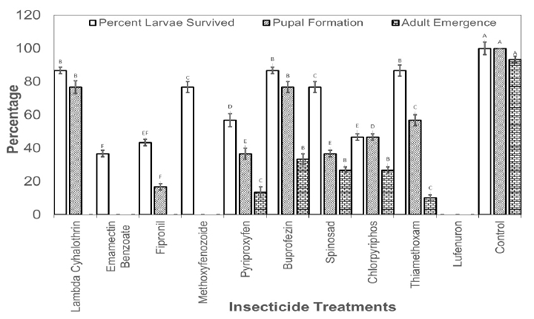
Figure 2: Percentage of third instar larvae those survived the effect of insecticides and emerged as adult insects.
The larvae those survived the toxicity of recommended dosed of the insecticides were also recorded and allowed to grow till adult insects. Results revealed that no larvae reached adult stage in Lambda Cyhalothrin, Emamectin Banzoate, Fipronil, Methoxyfenozoide, Pyriproxyfen, Buprofezin, Chlorpyriphos and Lufenuron. The larvae reached adult stage only in spinosad and Thiamethoxam treatments treated at second instar with the recommended field doses of the insecticides (Figure 1). As far as the treatment with the recommended doses of insecticide at third instar larvae is concerned, larvae reached adult stage in Pyriproxyfen, Buprofezin, Spinosad, Chlorpyriphos, and Thiamethoxam (Figure 2).
Discussion
In the present studies all the insecticide showed varying degree of effectiveness against second and third instar larvae of S. litura under laboratory controlled conditions. Some of the insecticides such as Emmamectin benzoate, Fipronil, Pyriproxyfen, Chlorpyriphos and Lufenuron proved highly toxic, whereas others such as Methoxyfenozoide, Spinosad, Buprofezin proved least effective. Insecticide such as Lambda Cyhalothirn and Thiamethoxam proved ineffective.
Members of the new chemistry insecticide such as Emamectin Benzoate which belongs to the avermectin group and act as an activator of the chloride channel (Teran-Vargas et al., 1997). Emamectin Benzoate seems to be highly effective insecticide as it caused high mortality even at 24 h post treatment and the insects showed no sign of resistance. Hence, it may be used effectively for the management of S. litura in the field.
The next highly effective insecticide was lufenuron which caused 100 percent mortality of the insects. Lufenuron is an IGR insecticide which is a chitin synthesis inhibitor and responsible for the incorporation of N-actylglucosamine into the chitin, causing formation of abnormal cutical and consequently cause death of the insect (Nakagawa et al., 1996; Nakagawa and Matsumura, 1994; Nakagawa and Matsumura, 1993). In some literature it has been documented that Lufenuron acts as Juvenile hormone (JH) in insects (Tunaz, 2004). In an earlier investigation, Lufenuron proved highly toxic to S. litura and caused 100 percent mortality of third instar larvae of S. litura three days post treatment in all the concentrations, 25, 50 and 75 ppm (Tarik-ul-Islam et al., 2015). Another IGR, Buprofazin, a chitin synthesis inhibitor in our investigation was least effective. In previous investigations Buprofazin caused weight reduction, extended larval and pupal development but did not caused significant mortality (Nasr et al., 2010). Methoxyfenozide which belongs to the new chemistry insecticide of Moult Accelerating Compound (MAC) against Lepidoptera (Smagghe et al., 2003). These compounds stimulates the natural hormone receptors after binding directly to them and cause lethal moult (Dhadialla et al., 1998). In current studies, methoxyfenozide proved low to moderate toxic depending upon concentration and exposure time.
Similar to our studies (Sabri et al., 2016) caused maximum mortality compared to other insecticides against second instar S. litura.
Another new chemistry insecticide, Emamectin Benzoate proved highly effective which caused 100 percent mortality of the third instar larvae of S. litura. Similar, to our results Emamectin benzoate proved highly toxic and caused 100 percent mortality of S. litura larvae. It was also declared as the most robust insecticide with very low chronic LC90 value (Khan et al., 2011; El-Sayed, 2011). High effectiveness of Emamectin Benzoate was also documented by Gupta et al., 2004 while comparing toxicity of conventional and certain new chemistry insecticides against five day old S. litura.
Chlorpyriphos proved highly effective compared to conventional insecticides namely, Lambda Cyhalothrin and Thiamethoxam, causing high mortality of more than 90 percent. Chlorpyriphos being a nerve poison and effector of nerve impulses show quick action against insect pests. In an earlier investigation it was found highly effective against S. litura (Ahmed et al., 2006) and no cross resistance was observed between methomyl and Chlorpyriphos proposing its high effectiveness against S. exigua (Argentine et al., 2002).
Conclusion
In our present studies, Emamectin Benzoate proved most effective, followed by Chlorpyriphos a conventional OP insecticide, whereas among IGRs Lufenuron proved most effective. The results will help us to devise new environmentally safe IPM strategies for the control of S. litura in agroecosystem.
Acknowledgements
The authors are very grateful for Kamran Mirza, Khizer Hayat and Muhammed Wasif for maintaining laboratory culture of Spodoptera culture throughout the year.
Conflict of Interest
The authors declare not conflict of interest.
References





