South Asian Journal of Life Sciences
Research Article
Population Dynamic and Characterization of Parachanna Obscura (Günter, 1861) in Kakum River (Ghana) and Lake Nokoué (Benin): Comparative Study
Mahunan Tobias Césaire Azon1*, Diane Nathalie Kpoguè2, Juste Vital Vodounnou2, Edmond Sossoukpè1, Emile Didier Fiogbé1
1Research Laboratory on Wetlands (LRZH), University of Abomey-Calavi (UAC), 01 BP 526 Cotonou, Benin; 2School of Aquaculture, National University of Agriculture (Eq/UNA), BP 43 Kétou, Benin.
Abstract | Parachanna obscura can be found in several wetlands such as flooded plains, lakes, rivers, shallows and ponds. However, its scarcity in water plans through latter years constitutes a major preoccupation for researchers. The current study aims to evaluate comparatively the population dynamic of Parachanna obscura in two West-African water plans: the Kakum River of Ghana and Lake Nokoué of Benin. This evaluation has been carried out based on parameters such as length-weight relationship, sex ratio, gonado-somatic and hepato-somatic indexes and the first maturity height of Parachanna obscura in Lake Nokoué (Benin) and Kakum River (Ghana). Our results revealed a negative allometric growth b = 1.06 and 1.62 (b < 3) respectively in specimens of P. obscura of Kakum River and Lake Nokoué. The male female sex ratio is based on males in Lake Nokoué though in the Kakum River, it’s based on females. The highest gonado-somatic index (0.92±0.2 % in February, 0.56±0.18 % in March and 0.82±0.17 % in April regarding females and 0.29±0.07 % in February, 0.29±0.08 % in March and 0.30±0.05 % in April regarding males) and hepato-somatic (0.82±0.16 % in February, 0.73±0.18 % in March and 0.81±0.24 % in April regarding females and 0.72±0.21 % in February, 0.77±0.19 % in March and 0.75±0.17 % in April regarding males) were recorded in specimens of Kakum River though the lowest GSI (0.78±0.27 % in February, 0.55±0.25 % in March and 0.64±0.24 % in April regarding females and 0.11±0.03 % in February, 0.14±0.06 % in March and 0.13±0.04 % in April regarding males) and HSI (0.5±0.23 % in February, 0.57±0.22 % in March and 0.61±0.25 % in April regarding females and 0.27±0.09 % in February, 0.39±0.22 % in March and 0.33±0.12 % in April regarding males) were recorded in Lake Nokoué. The first sexual maturity height in females of P. obscura was 15 cm TL and 12.5 cm TL respectively in specimens of Lake Nokoué and Kakum River.
Keywords: Parachanna obscura; Length-weight relationship; Sex ratio; Gonado-somatic index; Hepato-somatic index; First sexual maturity height; Lake Nokoué; Kakum River.
Editor | Muhammad Nauman Zahid, Quality Operations Laboratory, University of Veterinary and Animal Sciences, Lahore, Pakistan.
Received | January 12, 2019 Accepted | February 22, 2019; Published | June 01, 2020
*Correspondence | Mahunan Tobias Césaire Azon, Research Laboratory on Wetlands (LRZH), University of Abomey-Calavi (UAC), 01 BP 526 Cotonou, Benin; Email: azonmahunan@yahoo.fr
Citation | Azon MTC, Kpoguè DN, Vodounnou JV, Sossoukpè E, Fiogbé ED (2020). Population dynamic and characterization of parachanna obscura (günter, 1861) in kakum river (ghana) and lake nokoué (benin): comparative study. S. Asian J. Life Sci. 8(1): 11-18.
DOI | http://dx.doi.org/10.17582/journal.sajls/2020/8.1.11.18
ISSN | 2311–0589
Copyright © 2020 Azon et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Snakehead fishes (Baily, 1994) are fresh water perciforms belonging to Channidae family though the genus Channa is found in Asia and Parachanna in Africa. Two species of Parachanna are encountered in West-Africa. It concerns Parachanna obscura and Parachanna africana (Syndenham, 1976). Parachanna obscura is frequently sold in local markets and is one of the most expensive fishes. The fish is in great request due to its medicinal and rejuvenating virtue and is especially recommended to patients for quit recovery from serious illness or after deliverance (Mat Jais et al., 1998). The snakehead fish is characterized by a higher nutritive value than Carps and Tilapias in term of proteins and fatty acid (Sharma et Simlot, 1971; Daniel Ama-Abasi and Anthony Ogar, 2013). It’s present in many countries such as Benin, Burkina Faso, Cameroon, Central Africa, Chad, Côte d’Ivoire, Ethiopia, Gambia, Ghana, Guinea, Bissau Guinea, equatorial Guinea, Mali, Niger, Nigeria, Senegal, Sierra Leone, Sudan and Togo (Teugels, 2003). The species is characterized by an elongate body covered by cycloid scales. According to the research work of Bolaji et al. (2011) based on some aspects of biology of the species, P. obscura is a carnivorous fish and feeds mainly on insects, fishes and other invertebrates. The snakehead fish can be encountered in several wetlands such as flooded plains, lakes, rivers, shallows and ponds (Guseva, 1990). Nowadays, the scarcity of P. obscura in our water plans led to an important preoccupation. The few recent studies available on the ichthyofauna of continental water plans (Adite and Rudi, 1995; Lalèyè et al., 2003; Lalèyè et al., 2004) are essentially focused on the great water bodies. Thus, Laleye et al. (2004) and Montchowui et al. (2008) reported in their studies respectively based on the ichthyological diversity of Ouémé River (Benin) and on the biodiversity and structure of Lake Hlan (Benin) fish community that Channids were among the lowest diversified fish families in these ecosystems. The overexploitation of fishery resources is currently a reality. It manifests mainly by a situation of high decrease in daily captures despite the improvement of the performances fishing equipment (Lalèyè, 1995; Levêque and Paugy, 1999). According to Isangedighi and Umoumoh (2011) the sex ratio of Parachanna obscura is essentially based on females in the Itu Cross River system (Nigeria). Similar results were also obtained by Udoh and Daniel (2001) showing that females are more susceptible to captures than males. Isangedighi and Umoumoh (2011) reported that the smallest mature female observed in Itu Cross River measured 17.5 cm LT though males measured 23.4 cm LT. Concerning the length-weight relationship, an isometric growth was observed in females of Parachanna obscura (b=3.01) though in males, this growth was allometric negative (b=2.74) (Vodounnou et al., 2017). The current study aims to evaluate comparatively the population dynamic of Parachanna obscura in two West-African water plans: Kakum River of Ghana and Lake Nokoué of Benin. This study looks forward to monitor the population dynamic of P. obscura in West-Africa and to participate to its preservation.
Material and methods
Sampling Process and Study Area
Specimens of Parachanna obscura were randomly collected from fishermen in Lake Nokoué (Benin) especially in the area of Ganvié landing stage (Photo 1) and those of Kakum River (Ghana) from Abaasa village (Photo 2). Sampling collection was carried out during a three (03) months period from February to April 2019. Fifty (50) specimens all heights included though seventeen (17) in February, sixteen (16) in March and seventeen (17) in April were collected from each country for a total hundred (100) specimens. They were then packed in plastic iceboxes containing ice and brought to the Fisheries and Coastal Research Laboratory (FCRL) of the University of Cape Coast (Ghana). Samples were rinsed with fresh and clean tap water in the laboratory and preserved separately with alcohol 96° until analysis of diverse parameters.
Morphometric Analyses
The total length (TL), standard length (SL) and weight (W) of each specimen were measured separately by considering each type of sample. These measurements are defined as follow:
Total length (TL): Distance from the snout to the end of the caudal fin (cm);
Standard length (SL): Distance from the snout to the caudal peduncle (cm).
Length measurements were taken by using an ichtyometer (PENTAIR Aquatic Eco-Systems) though weights were taken by an electrical scale (OHAUS).
Sexes were determined after dissection of fishes and males were set apart from females in each sample group. Length-weight relationships were also determined in each group by the following formula:
W= aTLb (Ricker, 1973).
Where W is the total weight of the fish;
TL, the total length;
« a » is the intercept to (y) axis and “b” the allometric coefficient.
“b” values enabled us to give precision on the mode of growth observed in Parachanna obscura populations of Lake Nokoué and Kakum River.
The first sexual maturity height (height at which 50% of individuals are sexually matured) was also determined in each population of Parachanna obscura. Indeed, height classes were formed and percentages of matured individuals were calculated. Six (06) height classes were built from specimens of Lake Nokoué though in those of Kakum River, the number of height classes was three (03).
Concerning the gonado-somatic index (GSI), it was calculated by expressing in percentage the ratio of gonad weight by somatic weight (body weight minus gonad weight) of the fish.
GSI = GW*100/(BW – GW)
By the same way, the hepato-somatic index (HSI) was calculated by expressing in percentage the ratio of liver weight by somatic weight (body weight minus liver weight) of the fish.
HSI = LW*100/(BW – LW)
Where, GW = gonad weight,
BW = body weight and
LW = liver weight of the fish.
Statistical Analyses
The statistical analyses were carried out by using the Statistica software version 6. The sex ratio in each population was determined with the “χ2” test. Length-weight relationships were established and appreciated by a regression analysis. A one way analysis of variance (ANOVA) was used for the comparison of « b » to the value 3. Differences were considered at 5% significance threshold.
Results
Sex Ratio
Of the 50 specimens collected from Lake Nokoué, 28 (56%) were males though 22 (44%) were females. These results provide a male:female sex ratio of 1.27:1 that is in favor of males. In contrary, of the 50 specimens collected from Kakum River, 19 (38%) were males though females were 31 (62%). Thus, in Kakum River, the male:female sex ratio of Parachanna obscura is 1:1.63 based on females. Indeed, in Lake Nokoué, we noticed a dominance of males of Parachanna obscura though in Kakum River, females’ number was high.
Population Structure
Generally, the total length of individuals collected from Lake Nokoué ranged from 16.4 to 43.2cm though in Kakum River, it varied from 18 to 27.5cm. In relation to sex, the total length of males and females from Lake Nokoué varied respectively from 17.3cm to 43.2cm (125.8g to 642.4g total weight) and 16.4cm to 34.7cm (103.2g to 339.2g total weight) (Table 1) though in Kakum River, these heights ranged from 19.1cm to 26.1cm (110.3g to 203g total weight) (Table 2) in males and 18cm to 27.5cm (112g to 192g total weight) in females.
Table 1: Variation of sex ratio in relation to height classes in Lake Nokoué.
| Height Classes (cm) | Males (M) | Females (F) | Total | % M | % F | M:F |
| 15-20 | 1 | 7 | 8 | 12.5 | 87.5 | 1:7 |
| 20-25 | 3 | 4 | 7 | 42.86 | 57.14 | 1:1.3 |
| 25-30 | 4 | 9 | 13 | 30.77 | 69.23 | 1:2.2 |
| 30-35 | 6 | 2 | 8 | 75 | 25 | 3:1 |
| 35-40 | 12 | 0 | 12 | 100 | 0 | 1:0 |
| 40-45 | 2 | 0 | 2 | 100 | 0 | 1:0 |
Table 2: Variation of sex ratio in relation to height classes in Kakum River.
| Height Classes (cm) | Males (M) | Females (F) | Total | % M | % F | M:F |
| 15-20 | 6 | 7 | 13 | 46.15 | 53.85 | 1:1.2 |
| 20-25 | 12 | 19 | 31 | 38.71 | 61.29 | 1:1.6 |
| 25-30 | 1 | 5 | 6 | 16.67 | 83.33 | 1:5 |
In Lake Nokoué, the height class 35-40cm (12 individuals) (Figure 1A) was in majority in males. The minority
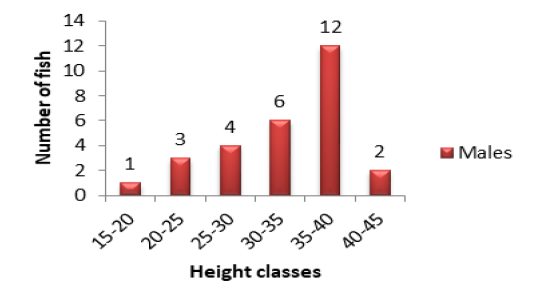
Figure 1A: Variation of specimen number in relation to height classes in males of P. obscura in Lake Nokoué.
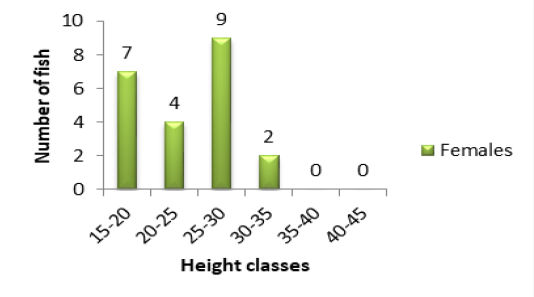
Figure 1B: Variation of specimen number in relation to height classes in females of P. obscura Lake Nokoué.
classes were those of 15-20cm (1 individual) and 40-45cm (2 individuals) (Figure 1A). The other classes were intermediary. In females, the major height class was 25-30cm (9 individuals) following by 15-20cm (7 individuals) (Figure 1B). There was no individual in 35-40cm and 40-45cm height classes (Figure 1B). Concerning the population of Kakum River, the major height class in males and females alike was 20-25cm (19 females and 12 males) following by 15-20cm (7 females and 6 males). The height class 25-30cm (5 females and 1 male) (Figures 2A and 2B) was in minority.
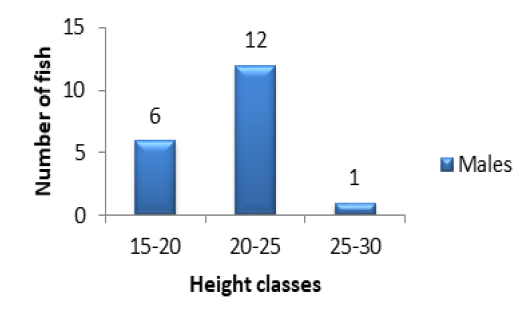
Figure 2A: Variation of specimen number in relation to height classes in males of P. obscura in Kakum River.
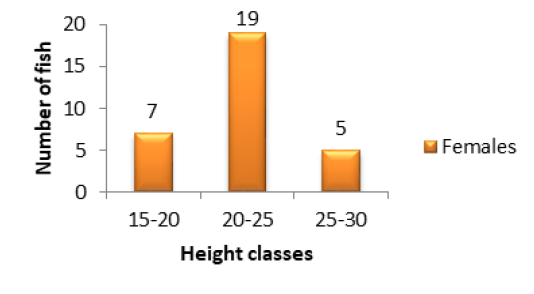
Figure 2B: Variation of specimen number in relation to height classes in females of P. obscura in Kakum River.
Sexual Maturity
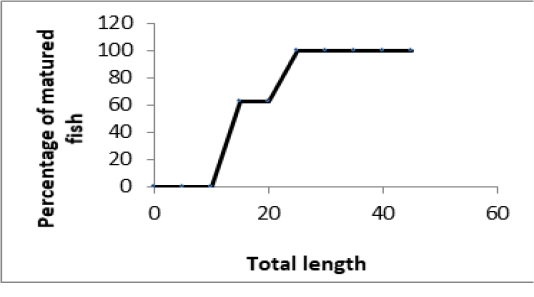
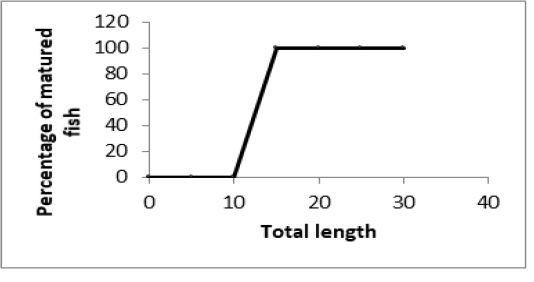
Generally, 50% of female specimens were sexually matured at 15cm TL (Figure 3A) in Lake Nokoué compared to those encountered in Kakum River where the maturity height was 12.5cm TL (Figure 3B).
Considering the gonado-somatic and hepato-somatic indexes, we noticed that they varied according to the sex and the medium.
The mean gonado-somatic index in females of P. obscura in Lake Nokoué varied from 0.78±0.27% in February, 0.55±0.25% in March and 0.64±0.24% in April though in males it varied from 0.11±0.03% in February, 0.14±0.06% in March and 0.13±0.04% in April. In Kakum River, the mean gonado-somatic index in females of P. obscura varied from 0.92±0.2% in February, 0.56±0.18% in March and 0.82±0.17% in April though in males, it varied from 0.29±0.07% in February, 0.29±0.08% in March and 0.30±0.05% in April.
Concerning the mean hepato-somatic index in females of P. obscura in Lake Nokoué, it varied from 0.5±0.23% in February, 0.57±0.22% in March and 0.61±0.25% in April though in males it varied from 0.27±0.09% in February, 0.39±0.22% in March and 0.33±0.12% in April. In the Kakum River, the mean hepato-somatic index in females of P. obscura varied from 0.82±0.16% in February, 0.73±0.18% in March and 0.81±0.24% in April though in males, it varied from 0.72±0.21% in February, 0.77±0.19 % in March and 0.75±0.17 % in April.
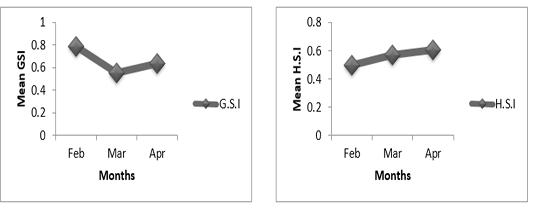
Figure 4A: Variation of gonado-somatic and hepato-somatic indexes in females of P. obscura in Lake Nokoué.
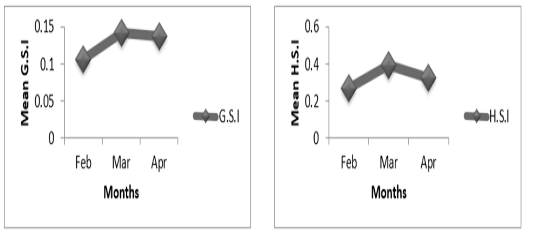
Figure 4B: Variation of gonado-somatic and hepato-somatic indexes in males ofP. obscura in Lake Nokoué.
The highest gonado-somatic and hepato-somatic indexes in females and males alike were recorded in specimens of Kakum River though the lowest were obtained in specimens of Lake Nokoué. Figures 4 and 5 show variations of gonado-somatic and hepato-somatic indexes in females and males of P. obscura in Lake Nokoué and Kakum River.
In females of P. obscura in Lake Nokoué, we noticed that the gonado-somatic index that was high in February, decreased in March and then increased in April (Figure 4A) though in males, the gonado-somatic index was crescent in February, March and then remained constant till April. The hepato-somatic index was also crescent except in males where we noticed a decrease during the month of April (Figure 4B). It’s important to mention that in specimens of Kakum River, the gonado-somatic and hepato-somatic indexes followed the same trends in females (Figure 5A) except in males where we noticed a decrease in the hepato-somatic index during the month of April (Figure 5B).
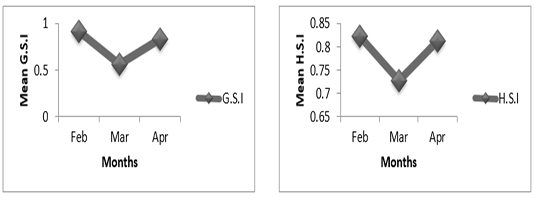
Figure 5A: Variation of gonado-somatic and hepato-somatic indexes in females of P. obscura in Kakum River.
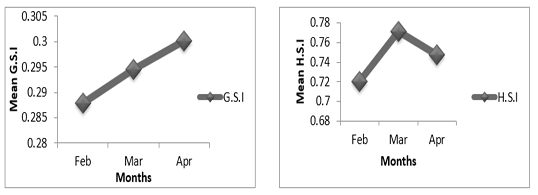
Figure 5B: Variation of gonado-somatic and hepato-somatic indexes in males of P. obscura in Kakum River.
Length-weight Relationship
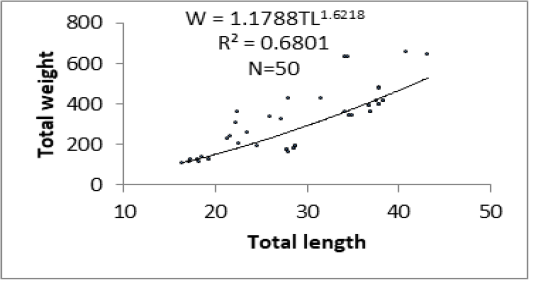
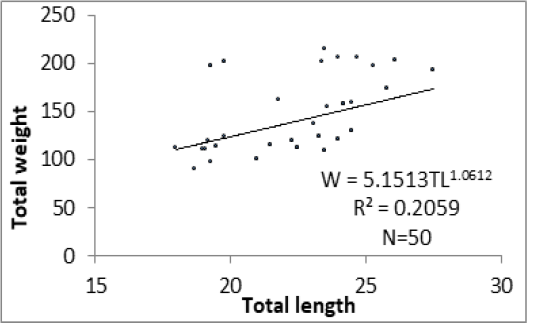
Figure 6 shows results of analyses between length-weight relationships of specimens of P. obscura in Lake Nokoué and Kakum River. Length-weight relationships were significantly different (p<0.05) with low values of R2 especially in specimens of Kakum River. Values of “b” varied from 1.06 to 1.62 respectively in specimens of Kakum River and Lake Nokoué. There is a significant difference (p<0.05) between “b” values in the length-weight relationships of both water plans. There is a negative allometric growth (b < 3) in specimens of P. obscura in both Kakum River and Lake Nokoué. This allometry is most accentuated in individuals of Kakum River than those of Lake Nokoué.
Discussion
Generally, we have collected both males and females individuals of P. obscura from Lake Nokoué and Kakum River. However, there was no external factor that could serve to distinguish sexes. The uro-genital papilla is similar in both males and females. Only the dissection enabled us to separate males from females through gonads observation. It’s also important to mention that the biggest specimens (654g body weight with 40.9cm TL) recorded during our study were males. These results corroborate with those of Isangedighi and Umoumoh (2011) who reported after observations on P. obscura that it is a mono-morphic teleost characterized by an absence of visual sexual dimorphism except males have biggest body weight than females.
The male:female sex ratio observed in Parachanna obscura in Lake Nokoué was 1.27:1. This ratio essentially based on males shows that these latter dominate on females in Lake Nokoué. These results are not similar to those of Isangedighi and Umoumoh (2011) and Odo et al. (2012) who observed a male:female sex ratio of 1:1.24 and 1:1.3 respectively in Itu cross River and Anambra River (Nigeria) during their studies on the general aspects of the biology of Parachanna obscura. In contrary, in Kakum River, the male:female sex ratio was 1:1.63; showing that females of Parachanna obscura dominate on males in Kakum River. These results are also similar to those of Udoh and Daniel (2001) who recorded a male:female ratio of 1:1.32 in favor of females. The preponderance of males of Parachanna obscura in Lake Nokoué could be a bad signal regarding the species sustainability because only males are not able to ensure the offspring. In addition, it’s noteworthy that males are most susceptible to captures than females due to the sex ratio is based on males. According to King (1991), the maintenance of a good population balance of a species depends on a sufficient quantity of females available to ensure the offspring. The scarcity of specimens of Parachanna obscura in continental water plans (especially in Lake Nokoué) is a synonyme of non-renewal of natural stocks associated to fishing pressure.
Regarding the population structure, there are significant differences (p<0.05) between height and weight of specimens (17.3cm to 43.2cm TL with 125.8g to 642.4g total weight in males) and (16.4cm to 34.7cm TL with 103.2g to 339.2g total weight in females) in Lake Nokoué on the one hand and those of Kakum River (19.1cm to 26.1cm TL with 110.3g to 203g total weight in males) and (18cm to 27.5cm TL with 112g to 192g total weight in females) on the other hand. Indeed, the biggest specimens of Parachanna obscura observed during the current study were obtained in Lake Nokoué. The weight of the biggest specimen obtained in Lake Nokoué was almost the triple of that of Kakum River. No female was observed from height 36cm until 45cm TL. These results are similar to those of Isangedighi and Umoumoh (2011) also Vodounnou et al. (2017) who reported respectively that the biggest specimens of Parachanna obscura encountered in Itu cross River (Nigeria) and Ouémé River (Benin) were males.
The first sexual maturity height observed in females of Parachanna obscura was 15cm and 12.5cm TL respectively in Lake Nokoué and Kakum River. These results don’t corroborate with those of Isangedighi and Umoumoh (2011) who reported that the smallest matured female observed in Itu cross River measured 17.5cm TL. According to Odo et al. (2012), this maturity height was 24.7cm TL. Thus, females of P. obscura in Kakum River earlier reach sexual maturity than those in Lake Nokoué. According to studies carried out by MacArthur and Wilson (1967), Pianka (1970) and Pierre François Verhulst (1838) on the different reproduction strategies, we can justify that P. obscura adopts the « r » strategy because specimens are early sexually matured. In addition, females encountered in Kakum River are smaller than those in Lake Nokoué. This difference observed in height can be explained by the fact that study media are different on the one hand and don’t have the same productivity on the other hand. This can be confirm by the results of Okyere et al. (2011) who reported that Kakum River is characterized by poor diversity of macro-zoobenthic fauna. This trend is a limiting factor to the development of fish community due to unavailability of food resources. In addition, the high pollution of the medium (Kakum River) featured by the presence of Chironomids larvae and Oligocheta in sediments (Okyere et al., 2011) doesn’t favor the primary production and so poor diversity of the macro-zoobenthic fauna that influences negatively fish growth. Other studies carried out by Laleye et al. (2006) and based on the biology of Synodontis schall and Synodontis nigrita Ouémé River (Benin) have also revealed a first sexual maturity height in females (16cm TL for S. schall and 22cm TL for S. nigrita).
Gonado-somatic and hepato-somatic indexes recorded in males and females specimens of Kakum River were higher than those obtained in specimens from Lake Nokoué. In addition, there are significant differences between gonado-somatic and hepato-somatic indexes of males and females not only in Lake Nokoué but also in Kakum River (p<0.05). Our results are lower than those obtained by Isangedighi and Umoumoh (2011) also Odo et al. (2012) (mean monthly GSI of 2.05±0.72%) with three picks in June, September and January. Low values of GSI (0.78±0.27% in February, 0.55±0.25% in March and 0.64±0.24% in April) and HSI (0.5±0.23% in February, 0.57±0.22% in March and 0.61±0.25% in April) obtained in the current study can be justified by the fact that fish samples were collected at the beginning of the rainy season. During this period of first rains, the productivity of aquatic media is relatively low. Indeed, most of females of P. obscura encountered were at pre-maturation stage. According to Victor and Akpocha (1992), the reproductive activity of P. obscura is more intensive in October and November.
The “b” values obtained in the length-weight relationships were 1.62 and 1.06 respectively in specimens of Lake Nokoué and those of Kakum River. These values are too lower than 3. Thus, the growth observed in P. obscura of both Lake Nokoué and Kakum River is allometric negative (b < 3). These results are lower than those obtained by Vodounnou et al. (2017) who reported a negative allometric growth (b = 2.74) in males of P. obscura in Ouémé River. Odo et al. (2012) also reported a negative allometric growth (b = 2.66; SD = 0.257) in specimens of P. obscura in Anambra River (Nigeria). Similar studies were carried out by Niyonkuru and Laleye (2012) on Lakes Nokoué and Ahémé (two Lakes of Southern Benin) and which were based on Sarotherodon melanotheron and Tilapia guineensis revealed also a negative allometric growth in both species (b = 2.85 and b = 2.87 respectively).
Conclusion
Our results revealed a negative allometric growth of P. obscura in Lake Nokoué and Kakum River alike. The sex ratio is based on males in Lake Nokoué and on females in the Kakum River. The first sexual maturity height in specimens of P. obscura in Lake Nokoué is higher than that recorded in specimens of Kakum River. Fishing pressures exerted by local consumers don’t enable a sustainable management of the species. Aquaculture remains the adequate solution for a good preservation of this species that constitutes a wealth for Africa. Other studies must be carried out for the mastery of P. obscura reproduction in captivity in order to guaranty a sustainable aquaculture of this species.
Acknowledgments
We give a big thank to the “Intra-Africa Academy Mobility Scheme” that granted us a six months PhD mobility in the University of Cape Coast (Ghana). We also address our kind regards to all the co-authors of this article for their diverse contributions.
Conflict of interest
There is no conflict of interest among the authors of this article.
authors contribution
Mahunan Tobias Césaire AZON: Sampling, data collection, statistical analyses and manuscript writing. Diane Nathalie Kpoguè: Advisor, manuscript correction, help in data analyses. Juste Vital Vodounnou: Advisor, manuscript correction, help in data collection. Edmond Sossoukpè: Advisor, help in the Length-Weight relationship analysis.Emile Didier Fiogbé: My PhD supervisor, manuscript correction and validation.
References