South Asian Journal of Life Sciences
Research Article
Antimicrobial Resistance of Salmonella Species Isolates from Broiler Birds in District Peshawar
Rafiullah1, Anwar Ali1, Muhammad Ijaz Ali1, Inamullah Wazir1, Naimatullah Khan2, Imtiaz Ali Shah1, Arifullah Khan3, Amin ur Rashid1
1Veterinary Research Institute Khyber Pakhtunkhwa, Peshawar, Pakistan; 2Abdul wali Khan university Mardan; 3Livestock & Dairy Development Department, Khyber Pakhtunkhwa, Pakistan.
Abstract | The present study was conducted in Veterinary Research Institute (VRI) District Peshawar from January, 2016 to August, 2016. A total of 200 samples were tested including 40 samples from each Liver, Kidney, Heart, Lungs and Intestines. Mac-Conkey agar was used for culture. The RapID-ONE-System was used for identification of Salmonella species. Different antibiotics, Ciprofloxacin, Gentamicin, Levofloxacin Ampicillin, Tetracyclin , and Azithromycin were used against Salmonella species. The prevalence rate was observed as S. gallinarum 63.33 % S. pullorum 35% and S. typhimurium 1.66 %. The most prevalence was found 35% in liver and 30% in intestines. Ampicillin was more resistant (76.66%) followed by Gentamicin 75%. While the highest sensitivity/susceptibility rate was 51.66 % observed by Levofloxacin. It was concluded from the current study that Salmonella have high resistance against certain antibiotics. and the drugs like Ampicillin showed reduced action against Salmonella infection. The resistance pattern of the drugs against Salmonella is uniformly changing therefore it is suggested that there may be a continuous research study to evaluate the resistance of the Salmonella and haphazard use of antibiotics may be discouraged.
Keywords: Salmonella , VRI, Resistance, Sensitivity, Prevalence, Antibiotics
Editor | Muhammad Nauman Zahid, Quality Operations Laboratory, University of Veterinary and Animal Sciences, Lahore, Pakistan.
Received | May 30, 2018 Accepted | August 04, 2018; Published | September 07, 2018
*Correspondence | Rafiullah, Veterinary Research Institute Khyber Pakhtunkhwa, Peshawar, Pakistan; Email: drrafi_ullah@yahoo.com
Citation | Rafiullah, Ali A, Ali MI, Wazir I, Khan N, Shah IA, Khan A, Rashid AU (2018). Antimicrobial resistance of salmonella species isolates from broiler birds in district peshawar. S. Asian J. Life Sci. 6(2): 46-53.
DOI | http://dx.doi.org/10.17582/journal.sajls/2018/6.2.46.53
ISSN | 2311–0589
Copyright © 2018 Rafiullah et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Salmonella is a gram negative, rod shaped , flagellated bacteria of the family Enterobacteriaceae. Salmonella is mostly found in the digestive tracts of reptiles, birds and mammals (A Karsi et al., 2008) Spores are not produced by Salmonella for the propagation of their breeding. The diameter of Salmonella is just about 0.7 to 1.5 µm, lengths from 2 to 5 µm. Salmonella is chemotropic in nature and gets energy from the redox reactions of organic compound. It has the capability of existing both in presence or absence of oxygen (A. Fabriga et al., 2013). As a result of fermentation Salmonella is competent of producing Hydrogen Sulphide gas (H2S) (M.Wierup et al., 2017).
Based on the specificity and clinical prototype, Salmonella can be categorized into different groups such as Salmonella typhimurium and Salmonella paratyphimurium A, B and C. similarly Salmonella dublin occurs in bovines, Salmonella typhimuriumsuis and Salmonella choleraesuis in swine, Salmonella pullorum and Salmonella gallinarum are found in birds (I. Gantois et al., 2009). Salmonella was first discovered by Karl Eberth in the spleens of typhoid patients 1880 (C. J. Eberth, 1880). Four years later in 1884 Georg Theodor Gaffky was the pioneer to successfully grow the pathogen in pure culture (A.Hardy, 1999). After an year a medical research scientist Theobald Smith discovered what would be later known as Salmonella enterica . The department was under the supervision of Daniel Elmer Salmon, a veterinary pathologist (FDA/CFSAN 2008).
In 1900 Joseph Leon Lignières suggested that the bacteria should be named as Salmonella due to the discovery of Daniel Salmon. The genus Salmonella comprises of two species, salmonella bongori and Salmonella enterica. According to the white-kauffmann-Le Minor scheme, Salmonella enterica is again divided into six subspecies (Salmonella enterica enterica, Salmonella enterica houtenae, Salmonella enterica arizonae, Salmonella enterica diarizonae, Salmonella enterica salamae, Salmonella enterica indica (P. Grimont et al., 2007).
Salmonella is a causative agent of various food borne diseases. The symptoms of Typhoid fever (Enteric fever) are diarrhea, fever, abdominal pain, and rashes after ingestion of Salmonella enterica serovar typhimurium. The mortality rate is 10-15% in cases of enteric fever in those regions where the sanitation system is very poor. Similarly, in case of Bacteremia the mortality rate is 8% if untreated. Gastroenteritis is another vital kind of salmonellosis which is caused Salmonella enterica serovar enteritidis and Salmonella enterica serovar typhimurium. Symptoms of the mentioned disease are appeared 6-8 hours after the use of unhygienic food or water, in the form of abdominal soreness, diarrhea and vomiting. The prevalence rate of typhoidal salmonellosis is less than that of nontyphoidal salmonellosis worldwide.
In developed countries of the world the mortality rate in case of Typhoid fever is 5 to 30% of the typhoid-infected persons. As per the report of World Health Organization (WHO) 16 to 17 million people are affected annually from Typhoid that leads to 600,000 deaths. The mortality rates among the typhoid infected individuals, differ from region to region, but can be as high as 5 to 7% in spite of the use of proper antibiotic treatment. In case of non-typhoidal salmonellosis the mortality rate is1.3 billion cases with 3 million deaths. 2 to 4 million people are infected with Salmonella out of which 500 deaths occur each year. Reports reveal that only 1 to 10% of cases are found in Africa, Asia, and South and Central America (C. F Pui, 2011, D. Hanes 2003, L. Hu et al., 2003).
Enteric fever (Typhoid fever) is prevalent in Asia, Africa, Eastern, Southern European countries, Middle East, South and Central America. Children are more susceptible to salmonella infection in Mekong Delta region of Vietnam. The most well-known epidemic of typhoid fever is Typhoid Mary (C. A Scherer et al., 2001). The epidemiology and antimicrobial resistance of Salmonella in Africa is underreported (A. G. Sow et al., 2007).
According to the report of Central Veterinary Laboratory (CVL), Harare the prevalence rate of S. enteritidis is rising frequently in chickens since 1993 (P.V. Makaya et al., 1998) A significant prevalence of S. enteritidis in both large-scale commercial (LSC) poultry and small-scale commercial (SSC) poultry has been reported in Zimbabwe. In South Africa, an increased incidence of S. enteritidis was reported after the first poultry-associated epidemic in 1991(J. Khumalo et al., 2014) reaching an incidence rate of 9.3% (223 incidents) between 1996 and 2006 (A. Kidanemariam et al., 2010) Chicken and associated products are considered to be the most important sources for salmonellosis.
Clinical Manifestation in Poultry Chickens
Salmonella infection is a burning issue not only economically but it also provides a gateway for secondary complications. Salmonella typhimurium is the most common serotype of Salmonella which plays a devastative role in poultry industry. It shows 40% of Salmonella isolates from 1950 to 1970 (W. J. Sojka et al., 1975). Poultry and poultry products are constantly considered the most important source for the transmission of Salmonella to human (M. Tietjen et al., 1995). From 1983 to 1987 it has been estimated in United States that poultry meat or eggs are the prominent agents of human salmonellosis (R. V. Tauxe, 1991).
Salmonellosis and Public Health
Domestic animals are infected from some of the salmonella serotype including poultry, sheep, and cattle. The status of the disease varying from acute to chronic which may leads to death. Some of the serotypes are strictly concerned to a specific host for example S. gallinarum and S. pollorum is concerned with poultry. These serotypes are associated with serious economic loss of the country (F. Calenge et al., 2010). Poultry industry facing a lot of problems by virtue of salmonellosis (A. G Fatma et al., 2012). A lot of preventive measurements have been taken since its discovery in order to manage commercial poultry farming.
However, Salmonellosis is also a burning economic issue in developing countries where the preventive protocols are not up to the mark and thus provides a route for the dispersal of these microorganisms (P. A. Barrow et al., 2011). The financial losses are primarily due to reduced growth rate, reduced feed conversion efficiency, morbidity, mortality, drop in egg production, hatchability and decreased fertility (S. K. Mamta et al., 2010).
Prevalence of Salmonellosis is enhancing day by day due to international trade and current travelling facilities which make it most vulnerable. Nowadays, the world is considered to be a global village which is correlated to one another and it becomes an easy passage for the transmission of salmonellosis among the different regions of the world (R. V. Tauxe et al., 2010).
Diagnosis
The investigation of Salmonellla can be carried out by widal test (manifestation of Salmonella antibodies against somatic (O) and flagellar antigen (H), but however the investigation is established by means of culturing technique). Culturing is one of the the most precise technique of investigation. Blood, tissue, bone marrow and stool are used to collect samples for the detection of salmonellosis. In the first week of the infection approximately 80% of the blood cultures examined becomes positive if no antibiotics are used. PCR can also be used for the diagnosis of Salmonella species whereas blood as used as a vital source for DNA extraction (A. V. Kumer et al., 2012).
Antibiotic Resistance
For the first time in 1960 Salmonella species shows resistance to antibiotics (K. Todar, 2005). Since then, the resistance to antibiotics for Salmonella serotypes becomes enhanced in the Saudi Arabia, United States, United Kingdom and other countries of the world. The main reason is the easy access and frequent use of the antibiotics which leads to the unfavorable consequences of resistance (T. J. Montville et al., 2008).
Salmonella enterica shows resistance to antibiotics such as ampicillin, chloramphenicol and trimthorpim-sulfamethoxazole (U. Grob et al., 1998). Swines and chicken eggs act as a carrier for Salmonella species (J. A. Crump et al., 2010). Antibiotics are nowadays frequently used for preventing diseases and promotion of growth cause an abrupt increase in propagation of human Salmonellosis (S. Singh et al., 2010). Indiscriminate and unwise use of antibiotics should be reduced in order to prevent the frequent transmission of human Salmonellosis (B.Yang et al., 2010).
Objectives
MATERIALS AND METHODS
Study Location and Time Frame
The current study was conducted in Veterinary Research Institute (VRI) District Peshawar from January 2016 to August 2016.
Sample Collection and Processing
A total of 200 broiler chicken samples of liver, kidney, heart, lungs and intestines were collected from the poultry post mortem section of Veterinary Research Institute (VRI) Peshawar to which the broiler were brought by different poultry farmers of the nearby areas. The samples were randomly collected in aseptic conditions and then transported under cold conditions to the Pathology and Bacteriology section of the Veterinary Research Institute (VRI) Peshawar for further processing. The collected samples were further processed on that very day.
Isolation of the Salmonella species
The Salmonella species were detected and isolated from the collected poultry samples using Mac-Conkey Agar. After the 24 hours of incubation period, the resultant cultured colonies of the samples were thoroughly observed for its colour. The plates having yellowish coloration were suspected to be positive and those with the colours other than yellow were thought to be negative.
For the morphology based identification of the Salmonella species Gram staining technique was used. The Salmonella species isolates from broiler chicken were also subjected to certain biochemical tests for the purpose of identification. For this purpose various tests were performed like catalase, oxidase, motility and citrate tests. The referenced manual which was followed for these biochemical tests was the Bergey’s Manual of Systemic Bacteriology (Krieg et al., 1984; Sneath et al., 1986). The detail account of these tests is follows.
Identification of Salmonella species Isolates through RapID-ONE System.
RapID-ONE System is a qualitative micromethod employing conventional and chromogenic substrates for the identification of the members of the Enterobacteriacae and other selected oxidase negative, gram negative bacilli. The tests used in the RapID-ONE System are based on microbial degradation of specific substrates detected by various indicator systems. The reactions employed are a combination of conventional tests and single-substrate chromogenic tests.
Antibiotic Susceptibility
The Salmonella isolates obtained from broiler chicken were then subjected to different antibiotics for checking its susceptibility/resistance against the antibiotics applied on them. The antibiotics were applied through Kirby buyer disk diffusion method.
For checking of the antimicrobial resistance of Salmonella species isolates from broiler chicken, sensitivity media was used. The inoculum of the Salmonella was prepared from the growth culture and then inoculated on the sensitivity media. The antibiotic disks were then positioned on the sensitivity media under aseptic conditions. The antibiotic disks were placed 10 mm apart from the edge of the media plate. The sensitivity media possessing the sensitivity disks were then subjected to incubation at 37°C for 24 hours. After the incubation process the inhibition zones produced by the antimicrobial disks were thoroughly observed and the diameters of the inhibition zones were recorded which were then matched with the Clinical and Laboratory Standards Institute (CLSI, 2014)
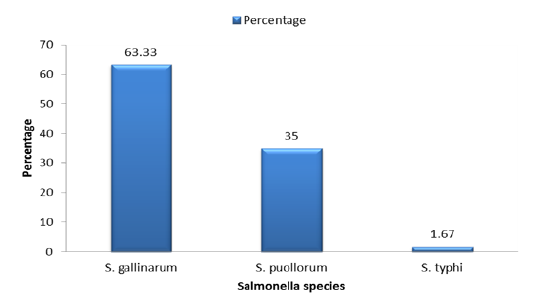
Table 3.1: Distribution of Salmonella species isolated from Broiler Chicken.
| S. No | Strain type | Number | Percentage |
| 01 | S. gallinarum | 38 | 63.33 |
| 02 | S. pullorum | 21 | 35 |
| 03 | S. typhimurium | 1 |
1.67 |
P= 0.3318 > .05 Non Significant
Table 3.2: Age wise Prevalence of Salmonella
| Sampling Group | Sample size | Positive cases | % of +ive Samples types |
P-Value |
||||
| Heart | Intestines | Liver | Lungs | Kidneys | ||||
| I | 120 | 41 | 12.19 | 29.26 | 34.14 | 9.75 | 14.63 | 0.0423 |
| II | 60 | 17 | 5.88 | 29.41 | 35.29 | 11.76 | 17.64 | 0.0106 |
| III | 20 | 02 | 00 | 50 | 50 | 00 | 00 | 0.0960 |
| Total | 200 | 60 | 06 | 18 | 21 | 06 | 09 | 0.1374 |
Note : Group I: 01-07 days, Group II: 8-14 days Group III: 15-21 days.
P= 0.1374 > .05 Non Significant
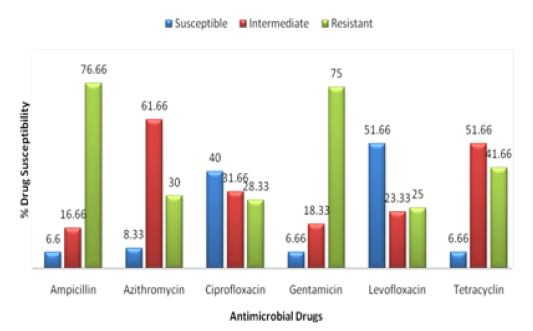
Table 3.3: Antibiotic Susceptibility Pattern of Salmonella species.
| S. No | Antimicrobial Drugs | Drug Concentration | No. of positive Samples | % Drug Susceptibility | P-Value | ||
| Susceptible | Intermediate | Resistant | |||||
| 01 | Ampicillin | 10 µg | 60 | 6.6 | 16.66 | 76.66 | 0.0982 |
| 02 | Azithromycin | 15 µg | 60 | 8.33 | 61.66 | 30 | 0.0525 |
| 03 | Ciprofloxacin | 05 µg | 60 | 40 | 31.66 | 28.33 | 0.0111 |
| 04 | Gentamicin | 05 µg | 60 | 6.66 | 18.33 | 75 | 0.0919 |
| 05 | Levofloxacin | 05 µg | 60 | 51.66 | 23.33 | 25 | 0.0354 |
| 06 | Tetracyclin | 30 µg | 60 | 6.66 | 51.66 | 41.66 |
0.0421 |
P= 0.0421< .05 Significant
RESULTS
This study was carried out for the identification of the Sal-
monella species isolated from different organs of the broiler chicken and also to analyze the resistance of the Salmonella against the action of certain drugs like Ampicillin, Azithromycin, Ciprofloxacin, Gentamicin, Levofloxacin and Tetracyclin. For this purpose 200 samples of various organs of the broiler chicken were collected from the Poultry Postmortem Section, Veterinary Research Institute (VRI), Peshawar.
Prevalence of Salmonella species Isolated from Broiler Chicken
Salmonella has many strains but the specific strains that were found in the current studies of the broiler chicken are Salmonella gallinarum, Salmonella pullorum and Salmonella typhimurium. Among these 3 strains of the Salmonella, the most prevalent strain was the Salmonella gallinarum (63.33%) and the least occurring strain that was found in this study was Salmonella typhimurium (1.67 %) but the intermediately found strain that was observed in the current study was Salmonella pullorum (35%) as shown in Figure 3.1,Table 3.1.
Age Wise Prevalence of Salmonella
In order to determine the relationship of the prevalence of Salmonella species infection with reference to their age, the suspected broilers chickens were divided into three different age groups. Age of the broiler present in Group I, II and III were 1-7, 8-14 and 15-21 days respectively. The sample size from group I was 120 out of which 41 (34.16%) cases were found positive for Salmonella infection. Among the 41 positive cases in Group I, the organ wise positive ratio that were found effected with Salmonella infection were heart 05 (12.19%), intestines 12 (29.26%), liver 14 (34.14%), lungs 04(9.75%) and kidney 06(14.63%).
In group II the sample size was 60 out of which 17 (28.33%) were observed positive for the Salmonella infection. In the same group, organ wise ratio of the Salmonella infection was found as such heart 01(5.88%), intestines 05(29.41%), liver 06(35.29%), lungs 02(11.76%) and kidneys 03(17.64%). Similarly in group III the number of the samples were 20 in which the rate of infection was found to be 02 (10%) out of which half of the infections were reported in liver and the other half in the intestines.
From the current study it can be revealed that among the three age groups, the broiler chicken in group I of age 1-7 days showed highest ratio of Salmonella infection than the other two groups. This may be due to the poor immune system development at this age which make them more vulnerable to the diseases and lesser ability to combat with foreign invaders. In overall sense, out of total 200 samples, 60 samples (30%) were positive for the Salmonella infection. Similarly the rate of infection among the different organs of the broiler chicken were found as such heart 06 (10%), intestines 18 (30%), liver 21(35%), lungs 06 (10%) and kidneys 09(15%), which showed that liver was more susceptible to acquire the infection by Salmonella and the least affected organs were observed to be heart and lungs as shown in Figure 3.2, Table 3.2.
Antibiotic Susceptibility/Resistance Pattern of Salmonella species.
The current study was more specifically designed to check the resistance offered by the Salmonella against certain drugs like Ampicillin, Azithromycin, Ciprofloxacin, Gentamicin, Levofloxcin and Tetracyclin. The pattern of resistance shown by Salmonella species against certain drugs/antibiotics were found by following Kirby-bauer disk diffusion procedure. According to the guidelines of the CLSI 2014, the response pattern of the Salmonella species against antibiotics was divided into three categories like Susceptible, Intermediate and Resistant.
The antimicrobial disks were applied to all the 60 positive samples, so the mentioned drugs Salmonella was found to be 6.6 % susceptible, 16.66 % Intermediate sensitive and 76.66% resistant against the drug Ampicillin. Similarly susceptibility pattern of the Salmonella against the drug Azithromycin was found 8.33 % Susceptible, 61.66% intermediate sensitive and 30% resistant. In the same way, Salmonella was found against Ciprofloxacin as 40% susceptible, 31.66 % Intermediate sensitive and 28.33 % Resistant. Against the Gentamicin, Salmonella were observed as 6.66% susceptible, 18.33% Intermediate sensitive and 75% resistant. The antibiotic Levofloxacin effect on Salmonella was noticed as 51.66% susceptible, 23.33% intermediate sensitive and 25% resistant. Salmonella was also studied under the action of the Tetracyclin and found it with such a behavior as 6.66% susceptible, 51.66 % intermediate sensitive and 41.66% resistant Figure 3.3, (Table 3.3).
So in short, the drug that was found more potent in this study was Levofloxacin as it is 51.42 % susceptible to the Salmonella species while the drug to which Salmonella showed the highest degree of resistance was found to be Ampicillin having resistant rate of 76.66 %. This may be due to overuse of the drug Ampicillin in poultry industry for certain infections which cause the Salmonella to become more resistant against this drug.
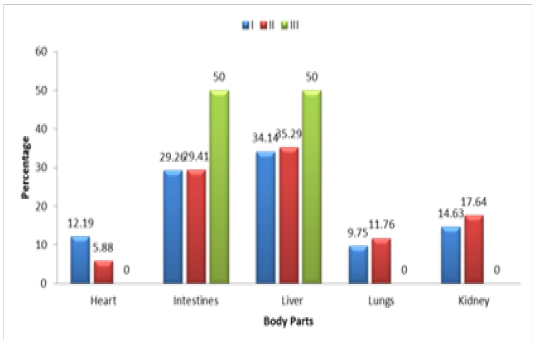
Figure 3.2: Age wise Prevalence of Salmonella
DISCUSSION
Salmonella is a considerable causative agent for Salmonellosis, which is a zoonotic disease. Besides being involved in certain medical issues, it do contribute in the contamination of the food reservoirs and industry. There are too many reasons for the food poisoning in human but Salmonella is also potent contributor for the said disease. (F.A. de Oliveria et al., 2010). In the year 2012, the presence of the Salmonella had frequently been reported in meat and other products of the poultry. In poultry industry, the emerging problem that is continuously spreading all over the world is the Salmonella infection. The world is now facing an economic loss in term of high death rate and retarded development of the poultry chicken. Salmonella is also a health problem for humanity (A. MacConkey 1905).
Salmonella are majorly observed in broiler chicken with a considerable ratio of 20-70% in many countries of the world. There are too many reasons for Salmonella being frequently present in the broiler meat, like the poor handling of the infected chicken intestines and contamination of the tools and equipments present in the poultry processing areas.
In the current study, the Salmonella prevalence was found to be 30% on the McConkey agar as a culturing media. These findings revealed a lesser rate of the prevalence then that of the results of the H.A. Shah and A.N. Korejo et al. 2012, (CDC 2010) which was reported as 48.75% in Pakistan at Tandojam, Sindh area. Again the findings of this study has lower prevalence ratio than the findings of the Arroyo et al. 2010, (Helms et al., 2002). It was 31.4% and also lesser than the prevalence observed by Vera et al. 2007, whose result was (58.6%) that was calculated from the occurrence of the Salmonella species in broiler chicken.
In the current study the percentage prevalence of Salmonella species for the different organs of the body are as such like kidneys 15%, liver 35% and heart 10% but the study of Vera et al. 2007, reported the percentage ratio for the same organs of the body as such like kidney 30%, liver 35% and heart 25% (A. H. Shah et al., 2012), which show somewhat similarities with the organ wise findings of the current study. The overall differences of this study with those of the other researchers may be contributed by different reasons like a changed sampling protocol, size of the samples collected, environmental conditions of the poultry farms, method adopted for the isolation of the Salmonella.
In these days antibiotics like beta-lactam are mainly used and in the same way new antibiotics are also added the routine treatment against various infections. Because of the over use of the beta-lactam antibiotics the main concern is the outbreaks of the serious issues like increased death rate, weak immune system and cost effective health treatment. The findings about the resistance pattern of different antibiotics will optimally control the health complications caused by the Salmonella by choosing the most appropriate drugs against it.
Many of the antibiotics such as Ampicillin, sulpha drugs and aminoglycosides have been observed to be less effective against Salmonella found in birds (S. Arroyo et al., 2010). The increased resistance has a devastating effect on health. Scientific studies have revealed that among all the antibiotics used 90 % found to be of under the therapeutic level (J. G. Vera, et al., 2007). In the current study, the resistance rate for the Salmonella was 76.66% but this was too much less than that of the Salmonella isolates from food items in Poland which was 93% resistant. Such a high resistance in the Salmonella against Ampicillin might indicate its exorbitant use in routine infections. In the current study some of the isolates obtained from the broiler chicken were found to be resistant to at least a single antimicrobial. There were also some isolates recorded in the broiler meat that can contribute for the food poisoning which are potent enough to be transferred from broiler meat to human (M. H. Mirmomeni et al., 2009), because some genes behave as quite resistant against diseases. Sometimes the invading bacteria form association with the gut bacteria and there may be a horizontal transfer of genes between the two. In the current study the peak resistance of Salmonella against Tetracyclin was recorded as 41.66% but the findings for the same drug was reported as 100% by Glenn et al this resistance may be developed by the reason that it is commonly used drug in veterinary practices and also used as a growth promoter in broiler chicken industry (M. H. Taddele et al., 2012).
The current study revealed that the percentage resistance of the Salmonella species against the quinolones antimicrobials like Ciprofloxacin was 28.33% while 25% resistance was observed for Levofloxacin. In comparison to the current study the resistance ratio of Salmonella against the same drug was reported as 3% in Malaysia but was 100% susceptible to the mentioned drug. The overall resistance of Salmonella against the quinolone class of antibiotic recorded low but still some incidence of the Salmonella has been observed in United States (T.W.R. Chia et al., 2009).
CONCLUSION
The flagellated bacteria of the family Enterobacteriaceae, Salmonella is mostly found in the digestive tracts of reptiles, birds and mammals and is a causative agent of various food borne diseases. Based on the current study it was observed that there is a considerable prevalence rate (30%) of Salmonella species in the broiler chicken. The prevalence rate of Salmonella in broiler chicken was observed as such as S. gallinarum 63.33 % S. pullorum 33% and S. typhimurium 1.66 %. The most prevalence was observed in liver 21 (35%) and intestines 18 (30%).
Antibiotics Ampicillin, Azithromycin, Ciprofloxacin, Gentamicin, Levofloxacin and Tetracyclin were used against Salmonella species among which Ampicillin faced more resistivity 76.66%, followed by Gentamicin 75%. While the highest sensitivity/susceptibility rate was 51.66 % shown by Levofloxacin. It was also found that some drugs like Ampicillin showed reduced action against Salmonella infection.
Recommendations
Acknowledgements
Contribution of all the authors is highly acknowledged.
Conflict of interest
There is no conflict of interest.
Authors Contribution
Rafiullah Anwar Ali Turi and Muhammad Ijaz Ali conceived the idea. Arifullah Khan, Naimatullah Khan and Imanullah Wazir collected samples. Rafiullah conducted whole study and trials with the help of Imtiaz Ali Shah & Ameen ur Rashid
REFERENCES