Journal of Infection and Molecular Biology
Review Article
Hookworm Infection: A Neglected Tropical Disease of Mankind
Muhammed Hossain*, Md. Jamal Uddin Bhuiyan
Department of Parasitology, Faculty of Veterinary and Animal Science, Sylhet Agricultural University, Sylhet-3100, Bangladesh.
Abstract | Hookworm infection is one of the neglected tropical disease which poses a global disease-burden by infecting 576 millions people around the world. Majority of hookworm infections are harbored by childern and adults. It is endemic in developing countries such as Asia, Africa, Southeast Asia, Bangladesh, Central and South America. Hookworm infection threats the mankind and cause anemia, hypo-albuminemia and malnutrition but other known effects include intellectual, cognitive impairment and stunted growth in children. Hookworm diagnosis is industrious as it has no gold standard diagnostic techniques, however, the ELISA shows moderate sensitivity and specificity to detect hookworm specific antibody titer in serum. Likewise, PCR method considered reliable for DNA detection in fecal samples.Lately, there is a drastic advancement in our realization about this widespread parasite. Advances in molecular biology had led to the identification of a variety of new molecules from hookworms, which have importance either in the molecular pathogenesis of hookworm infection or in the host-parasite relationship. Benzimidazole anthleminthics like albendazole, levamisole, mebendazole and pyrantel pamoate are the current corner stone for helminths treatment. This review aim to discuss currently published research on epidemiology, pathogenesis, clinical manifestations, diagnosis, treatment, immune mechanism, prevention and control.
Keywords | Hookworm, Neglected, Prevention and review
Editor | Tahir Yaqub, University of Veterinary and Animal Sciences, Lahore, Pakistan.
Received | September 09, 2016; Accepted | October 14, 2016; Published | November 07, 2016
*Correspondence | Muhammed Hossain, Department of Parasitology, Faculty of Veterinary and Animal Science, Sylhet Agricultural University, Sylhet-3100, Bangladesh; Email: bmhossain34sau@gmail.com
Citation | Hossain M and Bhuiyan MJU (2016). Hookworm infection: A neglected tropical disease of mankind. J. Inf. Mol. Biol. 4(2): 24-43.
DOI | http://dx.doi.org/10.14737/journal.jimb/2016/4.2.24.43
ISSN (Online) | 2307-5465; ISSN (Print) | 2307-5716
Copyright © 2016 Hossain and Bhuiyan. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Hookworm infection caused by Necator americanus and Ancylostoma duodenale are blood feeding nematode (Yulan et al., 2009) has been reported worldwide, especially among people in tropical and subtropical countries with low socio-economic status (Halpenny et al., 2013; Furst et al., 2012; Bethony et al., 2006). It is one of the most important parasites of soil-transmitted helminths (STHs) group. It has been estimated that 576 million people around the world are infected with hookworm in Sub-Saharan Africa, the Pacific Islands, India, Southeast Asia, Latin America and the Caribbean (De Silva et al., 2003a). Nowadays hookworm infection is among the most important tropical diseases in humans; the use of disability-adjusted life years as a quantitative measure of the burden of disease reveals that this infection out ranks African trypanosomiasis, dengue, Chagas disease, schistosomiasis and leprosy (Hotez et al., 2003). Several studies ensured its continuous existence in Bangladesh (Khair et al., 2016; Hossain et al., 2015; Sultana et al., 2012; Gilgen et al., 2001; Hall et al., 1994; Ali et al., 1985) along with strongyloidiasis (Hossain and Bhuiyan, 2016; Hossain et al., 2016). N. americanus has been found to be more predominant worldwide than A. duodenale (Jiraanankul et al., 2011). High-risk groups suffering from hookworm disease are children and pregnant women (Liabsuetrakul et al., 2009). Human acquires hookworm infection via third stage infective larvae (L3) penetrating the intact skin (Yulan et al., 2009; Liabsuetrakul et al., 2009; Tomono et al., 2003). The larvae migrate to heart and lung and then move to trachea where they have been swallowed. After going through two molts, larvae develop into blood feeding adult worms; where the female worms start to produce eggs that are excreted out through feces (Cline et al., 1984; Maxwell et al., 1987). The eggs hatch in moist soil and produce larvae that develop to the L3 stage after two molts, completing the hookworm life cycle (Craig and Scott, 2014; Bungiro and Cappello, 2004).
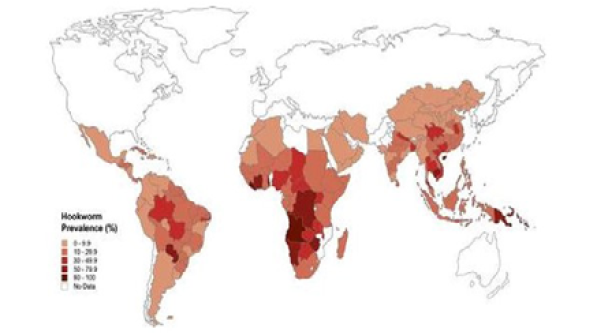
Figure 1: Global distribution of human hookworm infection [Illustration: Margaret Shear, Public Library of Science, adapted from (De Silva et al., 2003b)]
The hookworm infection mainly causes anaemia, hypo-albuminemia and malnutrition but other known effects include intellectual, cognitive impairment and stunted growth in children (Jardim-Botelho et al., 2008; Bethony et al., 2006; Brooker et al., 2006; Ekiz et al., 2005; Hotez et al., 2004; Sakti et al., 1999; Hotez, 1989; Albonico et al., 1998; Stoltzfus et al., 1997) and in pregnant women anemia supported by hookworm (Ndyomugyenyi et al., 2008). In previous decade prevention and control of hookworm infection has been globally neglected because most individuals with light to moderate hookworm infection generally are asymptomatic. The development of effective hookworm control is possible because of the availability of proven, cost effective and logistically feasible intervention strategies. Besides, for STH infections, regular periodic chemotherapy using benzimidazole anthleminthics of school-aged children delivered through the school system is the main intervention strategy (Hotez et al., 2005; Bundy et al., 2005; Awasthi et al., 2003) and also need to pay heed on sanitation and hygiene (Gruber et al., 2013; Bartram and Cairncross, 2010). Understanding where at risk populations live is fundamental for appropriate resource allocation and cost effective control. In particular, it allows for reliable estimation of the overall drug needs of programs and efficient geographical targeting of control efforts (Brooker and Michael, 2000). The school-based deworming programs could effectively reduce the hookworm infection of children (Wikagul et al., 2005; De Silva et al., 2003) but could miss positive adult cases. Considerable efforts need to develop a vaccine against hookworm and thus far more than 20 proteins have been explored as potential vaccine antigen targets (Bungiro et al., 2004; Hotez et al., 2008). However, there is still a long way to go before an effective hookworm vaccine might eventually become available. Metabolic profiling pursues a systematic biological approach and can deepen our understanding of metabolic responses of an organism to stimulate, such as disease, physiological changes and genetic modification (Nicholson et al., 1999). This review focused on current published research on improved diagnostic techniques for hookworm detection and immune mechanism thought to be responsible for infection along with epidemiology, pathogenesis, clinical manifestations, diagnosis, treatment, prevention and control.
Geographical Distribution
Hookworm is one of the important parasites of the soil-transmitted helminths group. Thus approximately one-half of the people of Southeast Asia living in poverty have one or more soil-transmitted helminths infection (Hotez et al., 2015). Necator americanus is the predominant hookworm worldwide, except in some defined locations where A. duodenale is focally endemic (Hotez et al., 2004). The distribution of worm burdens among different human hosts is highly over dispersed so that often only 10 percent of the infected population carries 70 percent of the worms (Bundy, 1995).
The major regions endemic for N. americanus include South and Southwest China (Hotez, 2002), South India (Yadla et al., 2003), Southeast Asia, sub-Saharan Africa, and Central and South America (Hotez et al., 2003) (Figure 1). Coastal areas of these regions are especially associated with high Necator transmission (Lwambo et al., 1992).
The A. duodenale predominant regions include more northerly latitudes of South and West China (e.g., Anhui, Sichuan Provinces) and India (e.g., Kanpur). The A. duodenale may survive in these harsher climates because of its ability to undergo arrested development in host tissues during periods of dryness or cold. A. duodenale infections also occur in Egypt, Northern Australia and in a few localities in Latin America including Northern Argentina, Paraguay (Labiano-Abello et al., 1999), Peru and in a region bordering El Salvador and Honduras (Knight et al., 1981).
HOST RANGE
N. americanus is generally considered the only member of this genus to infect humans. This species has also been recovered occasionally from non-human primates (Orihel, 1971; Michaud et al., 2003). It has been further suggested that the pig may serve as a transport host for N. americanus (Steenhard et al., 2000). A. duodenale is the only significant human hookworm of the genus Ancylostoma. Ancylostoma ceylanicum parasite of cats is infecting human as a zoonosis in some regions of Asia but it is not associated with host blood loss in humans (Carroll and Grove, 1986) and therefore is not considered as major pathogen (Hotez, 1995). High proportion of cases with A. ceylanicum infections, an unique zoonotic hookworm infection found in ASEAN countries, especially Malaysia, Thailand, Cambodia and Lao PDR (Ngui et al., 2012a; Ngui et al., 2012b). In northeastern Australia, the dog hookworm A. caninum has been reported to cause both eosinophilic enteritis and aphthous ileitis syndromes (Prociv and Croese, 1996; Landmann and Prociv, 2003). The natural history of zoonotic A. caninum infection has been extensively reviewed recently and will not be considered further (Prociv and Croese, 1996; Prociv, 1997). There is evidence that some persons are predisposed to a heavy (or light) hookworm burden owing to either genetic or exposure factors (Quinnell et al., 2001; Williams et al., 1997).
Epidemiological Patterns by Age and Sex
Children can be infected with hookworm as young as 6 month old (Brooker et al., 1999). The infection prevalence typically rises gradually with increasing age to adulthood (Shiferaw and Mengistu, 2015; Abossie et al., 2014; Wegayehu et al., 2013). Interestingly, recent evidence from studies of populations in China and Southeast Asia suggest that peak prevalence is observed among the middle aged or even individuals over the age of 60 years (Sengchanh et al., 2011; Lili et al., 2000; Gandhi et al., 2001; Bethony et al., 2002). In contrast, there appears to be considerable variation in age intensity profiles seen for hookworm (Bundy and Keymer, 1991). In West Africa, for example, convex age intensity profiles are observed (Udonsi, 1984; Behnke et al., 2000) while in China intensity increases continues to rise throughout life and is highest among the elderly (Gandhi et al., 2001; Bethony et al., 2002a).
An exception to this trend is the study in China, which shows that worm burdens tend to continuously increase with age (Ye et al., 1994). The observation that the elderly are at risk for heavy hookworm burdens has potentially important implications for the rapidly changing demographics in the developing world. It has been suggested that males are commonly infected with hookworm than females (Bundy, 1988a) which is suggested to reflect differential susceptibility to infection arising from immunosuppression associated with male hormones (Poulin, 1996; Moore and Wilson, 2002).
In particular, hookworm infection is more prevalent among adults occupational exposure is likely to be important where males are more commonly infected than females (Khair et al., 2016; Behnke et al., 2000) an observation in Mali explained by the fact that males are involved in constructing houses which frequently incorporate human faeces into materials to strengthen household structure. In contrast, in South China (Sengchanh et al., 2011; Gandhi et al., 2001; Bethony et al., 2002a) and Vietnam (Needham et al., 1998) females exhibit higher hookworm intensities among specific age groups. Because in Vietnam, elderly women were observed to be responsible for most of the night soil use and the higher infection levels could be explained, in this instance on occupational exposure (Humphries et al., 1997). However, Sex based differences in infection patterns may not always reflect occupational exposure. For example, in Hainan, China women exhibit a higher prevalence and intensity of hookworm compared to males despite the absence of night soil use (Abossie et al., 2014; Needham et al., 1998; Gandhi et al., 2001; Bethony et al., 2002).
Risk Factors
Agent Factors
Hookworms are nematodes belonging to the family Ancylostomatidae, a part of the super-family Strongyloidea. The two major genera that affect humans are Necator and Ancylostoma characterized by the presence of either teeth or cutting plates that line the adult parasite buccal capsule (Hotez, 1995). The two species that account for almost all human infections, A. duodenale and N. americanus are highly host specific and occur in most warm temperate regions (Beaver and Cupp, 1984). Developmentally arrested and can live in the soil for weeks if there is appropriate warmth, shade and moisture (Brooker et al., 2004) and can cause human infection. The surface protein of the organism is supposed to be antigenic and causes infection. The World Health Organization defines moderate intensity infections as those with 2000-3999 eggs per gram of feces and heavy intensity infections as those with 4,000 or more eggs per gram (Montresor et al., 2002). Whereas the intensities for the former peak in childhood and adolescence, hookworm intensity usually either steadily rises in intensity with age in adulthood (Hotez et al., 2004; Bethony et al., 2002). The biological basis for this observation is unknown (Olatunde and Onyemelukw, 1994).
Household, Socio-economic and Occupational Risk Factors
As the transmission of hookworm engages contamination of the environment by hookworm eggs, it is expected that risk factors for infection may include poor personal hygiene (Traub et al., 2004; Asaolu and Ofoezie, 2003; Gunawardena et al., 2005), low educational attainment (Mihrshahi et al., 2009; Liabsuetrakul et al., 2009) and household sanitation (Ensink et al., 2005) and unfinished house floor (Soares et al., 2011; Pullan et al., 2010), which in turn are influenced by differences in socio-economic status (Halpenny et al., 2013; Furst et al., 2012; Balen et al., 2011; Brooker et al., 2004; Traub et al., 2004). Some studies have demonstrated that hookworm infection is associated with the absence of a latrine (Hossain et al., 2015; Wang et al., 2012; Olsen et al., 2001; Chongsuvivatwong et al., 1996) and low socio-economic status (Holland et al., 1988). A study in Kenya showed that variation in household income and education level of the head of household were not associated with any helminths infection (Olsen et al., 2001) conversely a study in Nigeria exposed level of education is significantly associated with hookworm infection (Adeniyi et al., 2015; Quihui et al., 2006; Nematian et al., 2004). Hookworm has also been noted to be more common in families who are involved with agricultural pursuits, attributed to widespread use of faeces as night soil fertilizer (Humphries et al., 1997) and among vegetable growers and farmers (Kirwan et al., 2009; Conde et al., 2007; Hotez et al., 1997). When faeces are used deliberately, heavy fecal pollution of plantations occurs where few toilets are available resulting in high levels of hookworm infection (Schad et al., 1983). Foot ware is also risk factors for hookworm infection (Sandy et al., 2014; Alemu et al., 2003; Ratnayaka and Wang, 2012) because walking in barefoot have high chance of hookworm infection (Shiferaw and Mengistu, 2015; Abate et al., 2013; Alemu et al., 2011). For instance, in India, Bangladesh and Sri Lanka, high rates of infection are observed among workers and their families in the tea gardens (Hossain et al., 2015; Gilgen et al., 2001).
Seasonal and Environmental Factors
Since larval stages of hookworm for motility, rate of development and survival are dependent on the surrounding environmental humidity, temperature and ultra-violet light and geographical differences in transmission will be influenced by these factors and related factors such as rainfall, soil type and altitude (Bongi et al., 2005; Chandler, 1929). Chandler concluded that 20-30°C was optimal for transmission with larvae reaching maturity in five days, with the lower limit lying between 8-10°C and the upper limit 40-45°C. The above of temperatures 35-40°C arrests development and even death occurs (Nwosu, 1978; Udonsi and Atata, 1987; Smith and Schad, 1990) and at temperatures of 35°C larvae of N. americanus all dead, with the highest cumulative hatching rates obtained at 30°C (Udonsi and Atata, 1987).
The lower thermal limit of hookworm in the tropics is often determined by altitude KwaZulu-Natal Province, South Africa that hookworm transmission is confined to the coastal plain below 150 m above sea level (Appleton and Gouws, 1996; Appleton et al., 1999; Mabaso et al., 2003). Above these altitudes low temperatures (<20°C) limit the transmission of the parasite (Yu, 1995). It has been noted, that A. duodenale will occur in some areas where N. americanus L3 cannot survive during the winter months. It has been postulated that the unique ability of A. duodenale L3 to undergo arrested development in the human host, may allow this species to survive during the cold winter months (Schad et al., 1973). Since hookworms are unable to survive desiccation, the amount of rainfall in an area is also an important factor influencing hookworm transmission both spatially and temporally. The spatial relationship between rainfall and hookworm prevalence is well established (Brooker and Michael, 2000). In some endemic areas, hookworm infection exhibits marked seasonality (Khanum et al., 2014; Cook et al., 2009). For A. duodenale which undergoes arrested development following the transmission during the rainy season, new infections appear after 8-10 months (Schad et al., 1973).
Studies in West Africa show that populations of L3 larvae are highest during the rainy season (Udonsi et al., 1980) and faecal egg counts are highest 2-7 months after the rainy season (Knight and Merrett, 1981; Nwosu, 1981). However, although seasonal fluctuations in transmission occur, such fluctuations may be of little significance to the overall persistence of hookworm populations (Anderson, 1982). A final environmental factor that may influence the transmission of hookworm is soil type. It has long been believed that hookworm thrive in areas with sandy soils because of the small particle size and well aerated texture of sandy soil. So although infective larvae quickly die on the surface of sandy soil in direct sunlight, they are able to rapidly migrate into the soil and during the rains are able to migrate vertically as moisture permits. In contrast, clay soils inhibit larval migration. Early evidence of an association between soil type and hookworm was provided by studies in the southern American States (Brooker and Michael, 2000). High prevalence of hookworm was significantly associated with well drained sandy soil types (Mabaso et al., 2003; Saathoff et al., 2002) whereas low prevalence was associated with clay soils.
HISTORY AND MORPHOLOGY
In 1838, Dubini provided the detail description of the worm now known as A. duodenale after examining specimens taken from a woman who had died in Milan. Little importance seemed to be attached to these observations until 1880 when an epidemic of anaemia occurred amongst the miners digging the St. Gotthard tunnel. By 1903, the British Home Secretary fearing widespread health and no doubt economic effects on the national mining industry, commissioned a report on Ankylostomiasis in Westphalian Collieries. Hookworm infections were well established in the USA and in due course the Rockefeller Sanitary Commission for the eradication of hookworm disease was established (Hegner et al., 1938; Ettling, 1990; Crompton and Whitehead, 1993). The overall information of hookworm given (Table 1).
Table 1: Life history characteristics of human hookworm infections (Crompton & Whitehead, 1993)
|
Traits |
A. duodenale |
N. americanus |
|
Adult worm size (mm) |
||
|
Male |
8-11 |
7-9 |
|
Female |
10-13 |
9-11 |
|
Adult life span (years) |
1 |
3-5 |
|
Sex ratio (M:F) |
1:1 |
1.5:1 |
|
Pre-patent period (days) |
53 |
49-56 |
|
Fecundity (eggs/female/day) |
10000-25000 |
5000-10000 |
|
Optimum temp (°C) for free living larval stages |
20-27 |
28-32 |
|
Route of infection |
O, P, T |
P |
|
Arrested development |
Yes |
No |
Based on information from Hoagland & Schad (1978) and Beaver & Jung (1985); O: Oral route; P: Percutaneous route; T: Trans-placental route; Schad & Banwell (1984) review evidence to suggest that A. duodenale may also exploit the trans-mammary route of infection as does A. caninum (Miller, 1981)
Eggs of hookworms (A. duodenale and N. americanus) are similar in morphology. They are colorless and oval in shape, measuring about 65 x 40μm. The eggs contain an ovum which appears unsegmented (Sianto et al., 2009). In stool more than 12 hours old, larvae may be seen inside the egg. The L3 stage of larvae 600μm of length (Brooker et al., 2004).
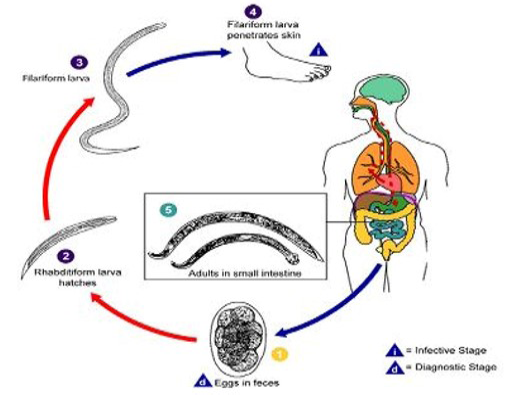
Figure 2: The biology of hookworm (Source: CDC)
TRANSMISSION
The highest rates of hookworm transmission occur in the world’s coastal regions, where infective third stage larvae can migrate freely in sandy soils and where temperature and moisture are optimal for viability of larvae (Mabaso et al., 2003). Acquiring hookworm infection is directly related to exposure to soil where filariform larvae, the infective stage live in and penetrate human skin (Liabsuetrakul et al., 2009; Tomono et al., 2003; Traub et al., 2004) when get opportunity (Figure 2). Human acquire hookworm when the infective larval stages (known as third stage larvae or L3) living in the soil either penetrate through the skin epidermis (Haas et al., 2005) (both N. americanus and A. duodenale) or when they are ingested (for A. duodenale) (Zeehaida et al., 2011; Olsen et al., 2009; Brooker et al., 2004). It has also been reported that N. americanus L3 will invade the buccal epithelium if they enter through the mouth (Nagahana et al., 1963). Exceptionally, larvae may be transmitted through fomites. For instance, if washed clothes are dried on the ground, larvae may creep on the textile from surrounding soil, resulting in an infestation when the piece of clothing is put on (Tomovic et al., 2008).
BIOLOGY OF HOOKWORM
The life cycle of A. duodenale was first elucidated by (Looss, 1901), and N. americanus was discovered in the Western Hemisphere (Chandler, 1929; Stiles, 1902). The life cycle of hookworms is direct (Hoagland and Schad, 1978; Schad and Banwell, 1984). Hookworms live in the small intestine and feed on host mucosa and blood (Roche and Layrisse, 1966). Female worms produce eggs which are passed out through stool (Augustine, 1922) to be embryonated in the soil. Under favourable condition (adequate but not excessive moisture; warmth, 25-28°C, shade) larvae hatch within 1 to 2 days. The rhabditiform larvae grow in the faeces in the soil (Cline et al., 1984) and after 5 to 10 days (and two molts) they become filariform (third-stage) larvae that are infective (Cort and Payne, 1922) and feeds on organic debris and microorganisms. These infective larvae can survive 3 to 4 weeks in favourable environmental conditions.
On contact with the human host, the larvae penetrate the skin (Logan, 2009) and are carried through the blood vessels to the heart and then to the lungs. They penetrate from the pulmonary capillaries into the pulmonary alveoli, migrate up the airways and pass down the esophagus through the stomach to the duodenum where the hookworms mature (Craig and Scott, 2014; Maxwell, 1987). Male locates female then they mate and eggs appear in the faeces. The L3 can live for several weeks in the soil until they exhaust their lipid metabolic reserves. Following host entry, the L3 receive a signal present in mammalian serum and tissue that causes them to resume development and secrete bioactive polypeptides (Hotez et al., 1993; Hawdon and Hotez, 1996). Resumption of development is cGMP dependent and involves a muscurinic neuronal pathway, which is similar to the one used for Caenorhabditis elegans dauer recovery (Hawdon and Datu, 2003). Among the major proteins secreted by host activated hookworm L3 is a zinc containing metallo-protease of the astacin class (Zhan et al., 2003) and two cysteine-rich secretory proteins known as ancylostoma secreted proteins, which belong to the pathogenesis related protein super family (Hotez and Hawdon, 1996; Hotez et al., 2003).
There are significant biological differences between the two major human hookworms (Hoagland and Schad, 1978; Hotez, 1995). N. americanus is smaller than A. duodenale and produces fewer eggs and causes less blood loss (Albonico et al., 1998). Therefore, some investigators believe that N. americanus more adept at immune invasion, produces less blood loss and better adapted to human parasitism (Hoagland and Schad, 1978; Pritchard and Brown, 2001). A. duodenale is associated with greater intestinal blood loss than any other hookworm. This accounts for the observation, best documented in Tanzania, that the species of hookworm being transmitted in a community strongly influences the burden of iron deficiency anaemia in the community (Albonico et al., 1998). However, N. americanus is more widespread worldwide and therefore, more significant as a cause of disease burden. Unlike N. americanus and A. duodenale also has the unique ability to undergo arrested development in humans (Ekiz et al., 2005; Schad et al., 1973) and may under certain conditions; enter human mammary glands during pregnancy prior to lactogenic transmission (Hotez, 1989; Yu et al., 1995). The occurrence of neonatal ancylostomiasis has been documented the best in Asia and Africa (Yu et al., 1995).
CLINICAL FEATURES
Acute Infection
The repeated exposure of N. americanus and A. duodenale L3 through the skin can result cutaneous syndrome known as “ground itch” (Brooker et al., 2004). Sometime sleep disturbance because of intense itching (Jackson et al., 2006). This comprises a pruritic erythematous papulo-vesicular rash. Ground itch appears most commonly on the hands and feet (Figure 3). In contrast A. braziliense L3 results in cutaneous larva migrants (CLM) which is characterized by serpiginous burrows appearing most frequently on the feet, buttocks and abdomen (Blackwell and Vega-Lopez, 2001). It is not known whether other animal hookworm such as A. caninum significant causes of CLM (Landmann and Prociv, 2003). The increased frequency of CLM among travellers returning from the Caribbean resorts and among residents along the Atlantic and Gulf coasts of the United States (Yosipovitch et al., 2002). A second form of CLM associated with folliculitis has also been reported (Sakai et al., 2008; Caumes et al., 2002).
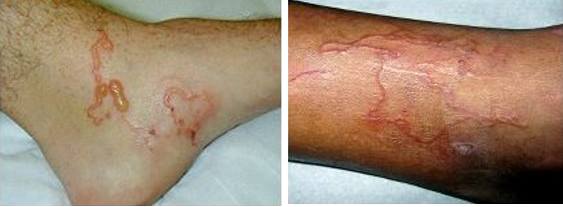
Figure 3: More exaggerated vesicular skin eruption by cutaneous hookworm larvae (Despommier et al., 2000)
Following the entry of human hookworm L3 undergo larval pulmonary migration, which can be accompanied by cough, sore throat and fever (Miller, 1979). Pulmonary hookworm infection resembles Loffler’s pneumonitis because of its association with lung eosinophil. Increased circulating levels of IgE occur in response to migrations of third stage larvae in the lungs and intestines (Maxwell et al., 1987). Hookworm pneumonitis is usually not severe although it may last for more than a month until the L3 leave the lungs and enter into the gastrointestinal tract following swallow. Entry of hookworm L3 entry into the gastrointestinal tract and their development into adult hookworms frequently results in epigastric pain (Anyaeze, 2003) which precedes the appearance of eggs in the faeces (Maxwell et al., 1987). When A. duodenale infection occurs via the oral route, the early L3 migrations sometimes produce a syndrome which is characterized by nausea, vomiting, pharyngeal irritation, cough, dyspnea and hoarseness (Harada, 1962).
Hypo-proteinemia caused by Hookworm
The consequences of hookworm infection are loss of protein (Betson et al., 2012) by plasma being directly ingested by the adult worm (Smith et al., 2010; Albonico and Savioli, 1997), hookworm rarely contributes to malnutrition with the important exception of iron deficiency anaemia. However, in some areas of high transmission with heavy worm burdens hookworm induced protein loss is substantial and may result in hypo-proteinemia leading to edema and even anasarca (Hotez, 2002). In addition, hookworm associated protein loss results in weight loss among vulnerable populations and consequently hookworm infected pregnant women in Sierra Leone show weight gain following treatment (Torlesse, 1999).
Hookworm Induced Anaemia
The major clinical manifestation of human hookworm infection is intestinal blood loss (Roche and Layrisee, 1966; Miller, 1979; Crompton and Stephenson, 1990). Heavy hookworm infections or moderate infections induce iron deficiency and microcytic hypochromic anaemia (Smith and Brooker, 2010; King et al., 2005; Lone et al., 2004; Beaver et al., 1984). The attachment of hookworms cutting organs to the intestinal mucosa and sub-mucosa and the subsequent rupture of intestinal capillaries and arterioles cause blood loss due to secretion of antiplatelet agents by the parasite helps to maintain continuous oozing of blood at the hookworm attachment site and the free flow of blood through the parasite alimentary canal (Stanssens et al., 1996; del Valle et al., 2003).
Table 2: Hookworm and host blood loss. The data are abstracted from (Holland, 1987, 1989; Pawlowski et al., 1991; Crompton, 2001) who give details of sources of information and techniques used to make measurements and estimates)
|
Traits |
A. duodenale |
N. americanus |
|
Intestinal blood loss in ml per worm per day, mean (range) |
0.15 (0.05-0.30) |
0.03 (0.01-0.04) |
|
Number of worms causing a blood loss of 1ml/day |
5 (4-7) |
25 (14-50) |
|
Blood loss (ml/day) per 1000 epg stool Mean±SD |
2.2 (1.54-2.86) 4.4±2.16 |
1.3 (0.82-2.24) 2.2±1.01 |
|
Iron loss (mg/day) per 1000 epg stool |
0.76-1.35 |
0.45-0.65 |
|
Worm burden responsible for 1000 epg stool |
11 |
32 |
The free hemoglobin is digested through the concerted action of aspartic, cysteine and metallo-hemoglobinases (Jones and Hotez, 2002; Williamson et al., 2003a; 2003b). Hookworm burdens of 40 to 160 worms are usually sufficient to cause anemia, although this depends on host iron stores (Lwambo et al., 1992) and the species of hookworm (Albonico et al., 1998). A strong association between intensity of infection and anaemia, with an increasing severity of anaemia at higher intensities of hookworm infection (Jackson, 1987; Sill et al. 1987; Shulman et al., 1996; Egwunyenga et al., 2001).Hookworm has traditionally been considered relatively unimportant in contributing towards the anaemia among pre-school children, where malaria is the important etiological factor to cause anemia (Brooker et al., 1999).
Perinatal Hookworm Infection
The hookworm anemia of pregnant women causes adverse maternal-fetal consequences including prematurity, low birth weight and impaired lactation (Miller, 1979). Hookworm infection during pregnancy has been reported to result in lactogenic transmission to neonate (Yu et al., 1995). This occurs because A. duodenale L3 can arrest their development in human tissues (Schad et al., 1973) with parturition the L3 enter the mammary glands and milk. Neonatal infection resulting from vertical transmission of hookworm results in severe disease associated with profound anaemia (Yu et al., 1995).
School Performance and Productivity in Adulthood
Severe and chronic infection with hookworm during children’s development also has consequences for their cognitive performance and ultimately their educational achievement (Jinabhai et al., 2001). Recent studies conducted throughout the developing world have provided evidence that school children infected with helminths perform poorly in tests of cognitive function (Shang, 2011; Jardim-Botelho et al., 2008; Watkins and Pollitt, 1997; Drake et al., 2000). The effect on cognitive function may occur as a result of combination of symptoms associated with infection, namely iron deficiency anaemia (IDA) and growth retardation (WHO, 2006; Grantham-McGregor and Ani, 2001; Mendez and Adair, 1999; Lozoff, 1990). A recent study among Bangladeshi tea pluckers found a negative association between intensity of hookworm and both hemoglobin and measures of labor productivity and that hemoglobin and productivity were positively associated (Gilgen et al., 2001).
Effects of Helminths in Allergic Patients
The global increase in allergy especially in urban areas (Pearce et al., 2007) has led researchers to propose a modified hygiene hypothesis in which the decline in helminths infection is associated with an increase in allergic diseases (Rook, 2009). A number of studies show that communities with helminths infection have reduced rates of allergy (van den Biggelaar et al., 2000; Cooper et al., 2003; Rodrigues et al., 2008) and the evidence that people with hookworm have less asthma (Leonardi-Bee et al., 2006; Scrivener et al., 2001; Flohr et al., 2010) has inspired researchers to use experimental infections on asthma patients (Feary et al., 2010). It is proposed that the active suppression of Th2 responses by helminths has a bystander effect on concurrent allergic responses (McSorley and Maizels, 2012). The other side of these phenomena is that anti-helminths treatment programmes risk increased rates of allergic disease and this has already been demonstrated in a number of intervention studies (Lynch et al., 1993; van den Biggelaar et al., 2004; Flohr et al., 2009).
DIAGNOSIS
There is no gold standard for diagnosis of hookworm infection and diagnosis is often delayed or overlooked due to patients presenting with non-specific gastrointestinal complaints. Nowadays widely taken samples for hookworm diagnosis are blood and fresh stool. Several diagnostic methods have been compared to detect the presence of hookworm including stool examination by Harada Mori filter paper culture, Kato-Katz thick smear, sodium acetate-acetic acid-formalin (SAF) solution, ether concentration method and the FLOTAC technique and Polymerase Chain Reaction (PCR).
Direct Smear
This method involves the identification of hookworm egg or larvae under microscope from fresh stool samples by normal saline, Eosin or Lugol’s Iodine as emulsifying agents (Cheesbrough, 1982) and Kato-Katz thick smear was prepared from each stool sample on microscope slides using 41.7 mg punched plastic templates (Katz et al., 1972), sodium acetate-acetic acid-formalin (SAF) solution (Bogoch et al., 2006; Marti and Escher, 1990), ether concentration method (Allen and Ridley, 1970) and the FLOTAC technique (Cringoli, 2006).
Cultural Techniques
Harada Mori culture techniques provide the morphological identification of hookworm larvae which first introduced by (Harada and Mori, 1955) then it is widely used (Vonghachack et al., 2015; Banu et al., 2013; Steinmann et al., 2007). This culture method is more effecitve than direct saline smear and ether concentration techniques (Marchi and Cantos, 2003).
Serology
Diagnosis of hookworm could be done by serological methods especially in patients with eosinophilia or mildly symptomatic patients. The serological methods determine the presence of hookworm antibody in the serum of the human hosts. The antibody could be determined by Enzyme Linked Immuno Sorbent Assay (ELISA) and Western Blot Analysis (WBA) (Brooker et al., 2004) as low titers of hookworm specific antibodies are noted in heavy infection along with a low or normal eosinophil count.
PCR
DNA extraction: Bead beating technique a conventional method for DNA extraction (Salonen et al., 2010) from raw stool samples but now sophisticated methods like QIAGEN DNeasy Blood & Tissue Kit are developed and used widely (Qiagen Inc., Valencia, CA).
Nested and Real Time PCR: The nested PCR method established for detection of hookworm. Purified DNA template was used for amplification in a DNA thermal cycler using a genus specific primer set as described by (Yong et. al., 2007). The rDNAregion comprising the first and second internal transcribed spacers plus the 5.8 S gene and approximately 50 nucleotides of the 28S rRNAwas amplified using oligonucleotide primers NC5 >: 5’-GTAGGTGAACCTGCGGAAGGATCATT-3’ (forward) and < NC2:5’-TTAGTTTCTTTTCCTCCGCT-3’ (reverse) designed to regions of the 18 S and 28 S genes, respectively (Gasser et al., 1996), and found to be conserved across a range of strong ylid nematodes. A real-time PCR method
Table 3: Treatment of hookworm [Modified from the Medical Letter on Drugs and Therapeutics, Drugs for Parasitic Infections (Anonymous, 2004). In children of 1-2 years the dose of albendazole is 200 mg instead of 400 mg, based on a recommendation in the Report of the WHO informal consultation on the use of praziquantel during pregnancy and lactation and albendazole/mebendazole in children less than 24 months (Kabatereine et al., 2007; Koukouknari et al., 2006; Montresor et al., 2003; WHO, 2002)]
|
Infection |
Drugs |
Dose |
|
|
Adult |
Child |
||
|
Hookworm |
Albendazole |
400 mg once |
400 mg once |
|
Mebendazole |
100 mg twice a day for 3 days |
100 mg twice a day for 3 days |
|
|
Pyrantel pamoate |
11 mg/kg (maximum dose 1gm) for 3 days |
11 mg/kg (maximum dose 1gm) for 3 days |
|
|
Levamisole |
2.5 mg/kg once; repeat after 7 days in heavy infection |
2.5 mg/kg once; repeat after 7 days in heavy infection |
|
developed by (Verweij et al., 2009) to detect hookworm DNA in fecal samples utilizing a primer and probe set.
TREATMENT
Benzimidazole anthleminthics are the current corner stone for helminths treatment because of their broad spectrum of activity, low cost, high efficacy and ease of administration (Savioli et al., 2002). Four anthleminthics are available for the treatment of hookworm such as albendazole, levamisole, mebendazole and pyrantel pamoate (Table 3). A single oral dose of ivermectin (200μg per kg body weight) kills the migrating larvae effectively (Caumes, 2003).
Varying cure and egg reducation rates have also been reported for levamisole and pyrantel pamoate (Utzinger and Keiser, 2004). The repeated round of pyrantel pamoate increases resistence to hookworm infection (Black et al., 2010). The anthelminthic treatment reduced intensities of hookworm and other helminths infection and improved haemoglobin among school children (Stephenson et al., 1989). Allergy of the patients misdirected anti-parasitic response of hypersensitive individuals (Artis et al., 2012). Not only children can benefit from treatment, in a recent study among pregnant women in Sierra Leone anthelmintic treatment increased haemoglobin concentration by 6.6 g/L, relative to controls (Torlesse and Hodges, 2000). In Sri Lanka treatment improved both the health of mothers and their birth outcomes (de Silva et al., 1999). Moreover, in areas where hookworm is endemic, reinfection often occurs within just a few months after deworming with the use of a benzimidazole anthelmintic (Albonico et al., 1995). In some cases, treatments are required three times a year to improve the iron status of the host (Stoltzfus et al., 1998; de Silva, 2003).
IMMUNE RESPONSES TO HOOK-WORM INFECTION
The complex life cycle of the hookworm offers numerous opportunities for the parasite and host to interact at the molecular level. Helminths infections are known to exert strong immune-modulatory effects on their mammalian hosts (Danilowicz-Luebert et al., 2011). Immunological responses to hookworm infection in both human and experimental animal hosts have extensively reviewed (Behnke, 1991; Loukas and Prociv, 2001). For instance, N. americanus will mature in hamsters but there is a wide change on the number of L3 that develop in adult hamsters (Rose and Behnke, 1990; Xue et al., 2003) and which frequently acquire resistance and do not develop patent infections (Rajasekariah et al., 1985; Xue et al., 2003). Likewise, A. duodenale will develop in dogs only with the administration of exogenous steroids (Leiby et al., 1987). This may be because the two species that account for almost all human infections, A. duodenale and N. americanus are highly host specific (Beaver et al., 1984). Hookworm based immune therapy resulted suppression of pro-inflammatory anti-gliadin immune-response and induction of systemic and mucosal type 2 cytokine response (Gaze et al., 2012) although overt suppression of clinical disease was not observed (Daveson et al., 2011).
The aspect of human immune response against hookworm infection is antibody levels to crude larval and adult soluble extracts or adult excretory/secretory products (Loukas and Prociv, 2001); often hookworm secretory/excretory products suppress intestinal colitis (Ferreira et al., 2013). The methods used to identify antibody responses (Sarles, 1938; Otto et al., 1942; Sheldon and Groover, 1942) and detailed analysis of Ig subclasses by enzyme-linked immune-sorbent assays (ELISA) and Western Blot analysis. These analyses have shown that extensive and vigorous antibody responses of all five of the human immunoglobulin (Ig) isotypes are mounted against crude antigen preparations in naturally infected individuals (Carr and Pritchard, 1987; Behnke, 1991; Loukas and Prociv, 2001). As antibody isotypes also differ in their biological properties, including their ability to mediate or block the killing of helminths (Shackelford et al., 1988; Khalife et al., 1989; Dunne et al., 1993).
Along with most helminths, the antibody response to hookworm consists predominantly of the Th2 antibody isotypes, IgG1, IgG4 and IgE with most attention on IgE because during hookworm infection serum levels of IgE increase 100-fold (Jarrett and Bazin, 1974). IgE participates in an orchestrated IgE network (Sutton and Gould, 1993; Garraud et al., 2003) with activation of this system often leading to cellular (mast cell, basophil and eosinophil) degranulation with toxicity against helminths (Garraud et al., 2003). The interpretation for the high levels of non-specific IgE found in the serum if an infected individual is a reduction in the risk of anaphylaxis (Hagan, 1993). The small proportion of the serum IgE that is raised against N. americanus is highly specific (Pritchard and Walsh, 1995) epitopes than other immunoglobulin isotypes.
During human intestinal infection with canine hookworm A. caninum, IgE responses proved to be more specific than the IgG responses to adult antigens with selected patients generating detectable levels of IgE but not IgG to a diagnostic A. caninum antigen (Jiz et al., 2009; Loukas et al., 1994).The IgG1 and IgG4 level are also elevated in hookworm infection with IgG4 against L3 suggested to be a marker of active infection with N. americanus (Palmer et al., 1996) and A. duodenale (Xue et al., 2000). The role of IgG4 is poorly understood although like IgE it is up regulated in atopic conditions and helminths allergic infections (Capron, 2011). It is thought to down regulate the immune response by competitively inhibiting IgE mediated mechanisms; i.e. blocking mast cell activation (Rihet et al., 1991) and IgG4 is stronger among other immunoglobulin (Geiger et al., 2011).
Adult hookworm also induces the production of secretory IgE, IgG and IgM but not IgA and the levels of these Ig return to normal after anthelminthic treatment. The absence of secretory IgA may reflect the secretion of hookworm proteases that specifically cleave IgA (Loukas and Prociv, 2001). Despite the extensive antibody response to infection, there is limited conclusive evidence that these antibodies offer any protection (Pritchard et al., 1995) by either significantly reducing larval or adult hookworm numbers, similar to that found for schistosome infections (Hagan et al., 1991; Dunne et al., 1992) and parasite burden because of the numerous uncontrolled variables such as exposure, behavioral modifications and co-infection with other helminths (Woolhouse, 1992; 1993).
The hallmark feature of the immune response to helminths infection is peripheral blood eosinophilia (Loukas and Prociv, 2001). Eosinophils predominate in the inflammatory responses to hookworm L3 in tissues and with sufficient larval dose, can be reflected in peripheral blood eosinophilia (Geiger et al., 2008; Behnke, 1991). Circulating eosinophils from human volunteers infected with N. americanus were functional and secreted superoxide (White et al., 1986). Peripheral eosinophil responses in experimental human infections with either N. americanus (Maxwell et al., 1987) or A. doudenale (Nawalinski and Schad, 1974) were boosted greatly by the arrival and development of worms in the gut and evidence suggests that these cells can kill infected larval stages but not the adults of most helminths species investigated (Meeusen and Balic, 2000). Mast cell degranulation in response to IgE allergen interaction plays a critical role in local mobilization and activation of eosinophils. While mast cell proteases degrade cuticular collagens of adult N. americanus (McKean and Pritchard, 1989) and are considered crucial to the host response to hookworms.
Eosinophilia, mastocytosis and IgE stimulation are the important three main immune alterations observed during a hookworm infection in humans. But the overall immune responses of human hosts to hookworm infection are remarkably similar to infections with other helminths; dominated by the production of T-helper2 (Th2) cytokines interleukin (IL-4, IL-5, IL-9, IL-10 and IL-13) which are consistent with the development of strong IgE, eosinophil and mast cell responses mentioned above. Therefore, the inherent ability of helminths to induce Th2 responses lead to elucidation of the underlying mechanisms in lung, skin or gut (Obata-Ninomiya et al., 2013; Harvie et al., 2013; Harvie et al., 2010; Knott et al., 2007) that lead to Th2 responses and in terms of understanding Th2 response function (MacDonald et al., 2002) along with increased mucus and fluid production to eject the parasites (Madden et al., 2004). A concomitant observation was the high level of IL-10 compared to other cytokines (IL-4, IL-5 and IL-13) that accompany chronic hookworm infection and decline that accompanies treatment (Turner et al., 2013; MacDonald et al., 2002) by using anthelmintic.
Experimental systems have demonstrated that the host protection to nematode infection may be a CD4+ T cell-dependent process with the IL-4 secreted by these cells has an essential or very important role in the process (Finkelman et al., 1997; Lawrence et al., 1998). Hookworm antigen induces cell apoptosis by intrinsic mitochondrial pathway and induces generation of suppressor CD4+ and CD8+ T cells (Gazzinelli-Guimaraes et al., 2013; Cuellar et al., 2009). However, the steps from IL-4 secretion to worm elimination are not still clear. Much of the basic cellular immunology of hookworm in humans remains to be done, especially the elucidation of the roles of cytokines, chemokine and cell surface markers during infection.
STRATEGIES FOR CONTROL AND PREVENTION
Because of its high transmission potential, hookworm has proven to be extremely difficult to eliminate or eradicate in areas of poverty and poor sanitation (Brooker et al., 2004). The interruption of transmission cycle is another key components of STH specially hookworm control (Hawdon, 2014; Strunz et al., 2014; Anderson et al., 2014; Truscott et al., 2014). The current strategy to control hookworm is chemotherapy although it alone cannot remove hookworm infection (Freeman et al., 2013) but with support of health education (Tomono et al., 2003), improved water and sanitation (Greene et al., 2012; Freeman and Clasen, 2011; Asaolu and Ofoezie, 2003) and socio-economic status (Mihrshahi et al., 2009). Beyond saying improved sanitation and hygiene are essential for the long term control of parasitic diseases. However, the availability of latrine facilities is associated with lower hookworm intensities (Chongsuvivatwong et al., 1996), the impact of introducing sanitation on infection levels may only be evident after decades (Esrey et al., 1991) and may not be completely effective. Despite associations between hookworm infection and use of footwear to protect from exposure to infective larvae, there is debate as to whether promotion of footwear is an effective control strategy (Albonico et al., 1999). The long time required for improved sanitation and behaviour change, for a quick-acting and medium term measure to control helminths infection is chemotherapy (Kabatereine et al., 2007; Koukouknari et al., 2006; Albonico et al., 1999) mentioned in (Table 3). The use of anti-hookworm vaccine with anthelmintic drugs brought benefits (Hotez et al., 2003). Efforts led by the World Health Organization have focused on annual, twice-yearly or thrice-yearly doses in school because the heaviest intensities of STH infections are most commonly encountered in school-age children (World Bank, 2003). Treatment of potable water also can reduce the transmission of hookworm (Clasen et al., 2007).
CONCLUSION
Hookworm infection is still a topic of great concern because of its high morbidity. The public health importance of hookworm infections are very little of practical significance is known about the details of how the parasites interact with their hosts including the nature and effectiveness of the immune responses that are generated. Interim it is warranted to investigate the risk factors involved in hookworm infection and screen patients from endemic areas prior to receiving chemotherapy. It is also essential to highlight that plethora of prevention effort in endemic countries such as health education campaigns on the disease, proper sanitation through appropriate disposal of fecal material, regular deworming and the use of protective footwear are achievable goals for reducing the occurrence of hookworm.
Acknowledgments
The authors acknowledge the contributions of his fellow research team for their sincere suggestion during the writing of whole manuscript.
Conflict of interests
The Authors declares no conflict of interest.
Authors’ contribution
The first author M Hossain did data management task, wrote the whole manuscript and made all the relevant correction during revision. MJU Bhuiyan helped in the supervision of the whole manuscript, and suggested for relevant correction of common error in written manuscript.
REFERENCES





