Journal of Infection and Molecular Biology
Research Article
Antimicrobial Susceptibilty Profile of Bacterial Pathogens in Surgical Site Infections at a Tertiary Care Hospital in Rawalpindi, Pakistan
Amaila Qaisar1*, Naeem Akhtar1, Rao Waqas Akhtar2, Waqas Latif2
1Rawalpindi Medical College, Rawalpindi; 2University of Health Sciences, Lahore, Pakistan.
Abstract | Surgical site infections (SSIs) results in a significant morbidity and mortality throughout the world. Here we determine the spectrum of bacterial isolates and their susceptibility patterns causing SSIs at Holy Family Hospital, Rawalpindi. A total of 100 pus swabs or pus were collected and subjected to microbial identification. Out of 100 pus swabs, 60 (60%) had positive aerobic bacterial growth corresponding to 8 different types of pathogenic bacteria. Escherichia coli (40.7%) was the most common pathogen followed by Pseudomonas aeruginosa (26.31%) and Staphylococcus aureus (19.73%). Gram negative bacteria were found to be more susceptible to Meropenem while it was Cefoxitin against Gram positive isolates. The study conclude an alarming increase of SSIs in this tertiary care hospital. In order to reduce the risk of post-operative surgical site infection, improvements in antibiotic prophylaxis including the timing of initial administration, appropriate choice of antibiotic agents, proper sterilization, improvement of operation theatre and ward environments is needed.
Keywords | Surgical site infections, Bacterial pathogens, Antimicrobial susceptibility profile
Editor | Tahir Yaqub, University of Veterinary and Animal Sciences, Lahore, Pakistan.
Received | May 28, 2015; Revised | June 29, 2015; Accepted | June 30, 2015; Published | July 19, 2015
*Correspondence | Amaila Qaisar, Rawalpindi Medical College, Rawalpindi, Pakistan; Email: amaila.qaiser134@gmail.com
Citation | Qaisar A, Akhtar N, Akhtar RW, Latif W (2015). Antimicrobial susceptibilty profile of bacterial pathogens in surgical site infections at a tertiary care hospital in Rawalpindi, Pakistan. J. Inf. Mol. Biol. 3(3): 57-61.
DOI | http://dx.doi.org/10.14737/journal.jimb/2015/3.3.57.61
ISSN (Online) | 2307-5465; ISSN (Print) | 2307-5716
Copyright © 2015 Qaisar et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Surgical site infections (SSIs) are one of the most common hospital-acquired infections, causing higher patient mortality and significantly longer length of stay (Afifi et al., 2009). About 77% of the deaths of surgical patients has been related to surgical wound infection (Mangram et al., 1999). SSIs account for 14% to 17% of all hospital-acquired infections and 38% of nosocomial infections in surgical patients. The Centers for Disease Control and Prevention (CDC) estimates that SSIs complicate approximately 5% of the nearly 30 million surgeries performed each year (Weigelt et al., 2010). Infection in a wound is a manifestation of disturbed host-bacteria equilibrium in favour of bacteria. This not only shows a systemic septic response but also inhibits the multiple processes that are involved in the wound healing (Awan et al., 2011). Wound infection is also the commonest and most troublesome disorder of wound healing. Post- operative wound infection has been a problem since surgery was started as a treatment modality (Ahmed et al., 2007). Advances in control of infections have not completely eradicated the problem because of development of resistant; an infected wound complicates the post-operative course and results in prolonged stay in the hospital and delayed recovery (Hunt, 1981). All surgical wounds are contaminated by bacteria, but only a few demonstrate clinical infection. SSIs are a consequence of several factors; the inoculum of bacteria introduced into the wound, the microenvironment of each wound and the integrity of the patient’s host defence mechanisms (Medeiros et al., 2005). The incidence of SSIs differ from one country to another and from area to area according to the different systems employed for the epidemiological control of hospital infections (Ercole et al., 2007). So, this study was designed to determine the clinical and microbiological profile of postoperative surgical site infections (SSIs) in patients undergoing general surgical operations in surgical wards of Holy Family Hospital.
This study was conducted at Holy Family Hospital Rawalpindi, Pakistan. A total of 100 Pus specimen were collected using sterile cotton swab from different patients admitted from October, 2012 to March, 2013. All the samples were collected from hospitalized patients in the department of surgery and internal medicine. The collected swabs were inoculated onto freshly prepared selective media like MacConkeys agar (without crystal violet and bile salts) and Blood agar for the isolation of the microorganisms. The plates were then incubated at 37ºC for 24 hours. Suspected isolates were presumptively identified by colony morphology, Gram staining along with biochemical tests (catalase, coagulase and oxidase) and pigment formation for Pseudomonas aeruginosa (pyocyanin and pyoverdine) as described by (Hassanzadeh et al., 2009).
P. aeruginosa isolates were confirmed by certain biochemical tests including oxidase test, citrate utilization and nitrate reduction test. While Escherichia coli and other Gram negative isolates were confirmed by other biochemical tests like Indole test, citrate utilization and triple sugar iron (TSI). For confirmation of the Gram positive organisms Catalase, Coagulase and DNAse test was performed. For Streptococcus pyogenes, bacitracin test and Hippurate hydrolysis test was performed.
Antimicrobial susceptibility testing of the isolated organisms was done by Kirby-Bauer disc diffusion method on Mueller-Hinton agar to determine their susceptibility pattern against commonly used antibiotics, following the standards of the Clinical Laboratory Standard Institute (CLSI) (Wayne, 2000). Cefoxitin was included in our panel of antimicrobial agents to detect possible methicillin-resistant S. aureus (MRSA) according to CLSI recommendations (Wayne, 2007). Implanted antibiotics were Ampicillin (10 µg), Augmentin (30 µg), Amikacin (15 µg), Bacitracin (10 units), Cefotaxime (30 µg), Cefoxitin (30 µg), Ceftazdime (30 µg), Colistin Sulphate (10 µg), Co-trimoxazole (25 µg), Erythromycin (15 µg), Fusidic Acid (10 µg), Levofloxacin (5 µg), Linezolid (30 µg), Meropenem (10 µg), Novobiocin (30 µg), Sulzone (10 µg) and Tetracyclin (30 µg ). Plates were incubated at 37ºC overnight aerobically. The interpretation was done as per CLSI guidelines 2013.
Data were analysed using SPSS 20.0 statistical software. Logistic regression analysis was performed to measure association of outcome with each independent variable; odds ratios (OR) and 95% confidence intervals (CI) were calculated for each risk factor (Table 1).
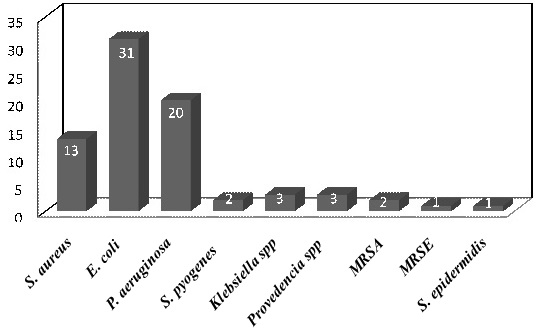
Out of 100 surgeries, 60 (60%) cases developed surgical site infections. Maximum number of patients (62%) were from 2nd, 3rd, and 4th decade of age group. Higher infection rate was noted in males 39 (65%) as compared to females 21 (35%). Out of hundred surgeries 46 (46%) were elective and 54 (54%) were emergency surgeries. 19 (19%) were done by consultant, most 65 (65%) done by PGT and least were done by HO 16 (16%). Most surgeries were from abdominal region 66 (66%) followed by limbs 20 (20%) and least 2 (2%) from chest region. A total of 8 different types of pathogenic bacteria were isolated along with different percentages (n=76). Out of seventy six (76) clinical isolates, 31 (40.7%) were E. coli followed by 20 (26.3%) P. aeruginosa, 13 (17%) S. aureus, Klebsiella spp. and Provedencia 3 (3.9%), S. pyogenes and methicillin-resistant S. aureus (MRSA) 2 (2.6%). Least were of S. epidermidis and MRSE 1 (1.3%) (Figure 1).
Table 1: Risk factor associated with surgical site infections
|
Variables |
Samples |
Positive |
P-Value |
Odd ratio (95%CI) |
|
Gender |
||||
|
Male |
57 |
39 |
0.12 |
1.91(0.83-4.4) |
|
Female |
43 |
21 |
||
|
Age Groups |
||||
|
0-20 Years |
18 |
10 |
||
|
20-40 Years |
47 |
28 |
0.50 |
1.47(0.47-4.6) |
|
40-60 Years |
24 |
15 |
0.94 |
1.05(0.27-3.9) |
|
>60 Years |
11 |
7 |
0.44 |
0.53(0.10-2.6) |
|
Surgeons |
||||
|
Post graduate trainees |
65 |
38 |
||
|
House officers |
16 |
10 |
0.95 |
0.96(0.28-3.2) |
|
Consultants |
19 |
12 |
0.37 |
1.66(0.54-5.1) |
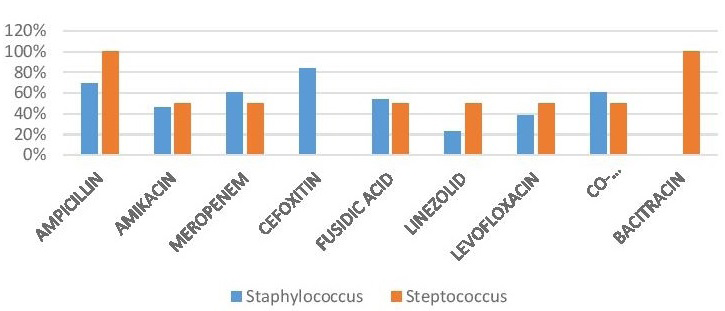
Among the studied antibiotics the most effective drugs against the gram positive isolates were Ampicillin 11 (84%) and Cefoxitin 11 (84%), followed by Meropenem 9 (55%), Co-trimoxazole 9 (55%), Fusidic acid 8 (52%), Amikacin 7 (48%), Levofloxacin 6 (44%), Linezolid 4 (36%) and Bacitracin 2 (100%), whereas they were resistant to Amikacin (53.33%), Levofloxacin (60%), and Linezolid (73.33%) (Figure 2) (Table 2).
Table 2: Percent antibiotic susceptibility pattern for Gram positive cocci isolated from SSIs
|
Antibiotics |
Bacterial isolates |
Sensitivity (%) |
|
|
S. aureus (n=13) |
S. pyogenes (n=2) |
||
|
Ampicillin |
9(69%) |
2(100%) |
11(84(%) |
|
Amikacin |
6(46%) |
1(50%) |
7(48%) |
|
Meropenem |
8(61%) |
1(50%) |
9(55%) |
|
Cefoxitin |
11(84%) |
- |
11(84%) |
|
Fusidic Acid |
7(54%) |
1(50%) |
8(52%) |
|
Linezolid |
3(23%) |
1(50%) |
4(36%) |
|
Levofloxacin |
5(38%) |
1(50%) |
6(44%) |
|
Co-Trimaxazole |
8(61%) |
1(50%) |
9(55%) |
|
Bacitracin |
- |
2(100%) |
2(100%) |
In case of gram negative isolates the most effective drugs were Augmentin 17(48%) and Meropenem 37 (71%) followed by Ceftazdime 15 (46%), Cefotaxime 17 (38%), ciprofloxacin 32 (61%), Co-trimoxazole 28 (51%), Sulzone 33 (62%), Levofloxacin 28 (39%), Colistin 15 (75%), Tetracyclin 7 (35%) and Amikacin 9 (45%). These isolates were resistant to Meropenem (35.08%), Ciprofloxacin (43.85%), Co-trimoxazole (50.87%) and Levofloxacin (50.87%) (Figure 3) (Table 3).
Surgical site infections are considered to be surgical complications that affect tissues, organs and cavities that have been manipulated or have suffered incision during a surgical procedure. In countries where resources are limited, postoperative surgical site infections remain as one of the major types of post-operative nosocomial infections (Allegranzi et al., 2011). The infection rate within a health care institution is a clinical indicator that helps in the evaluation of the quality of service delivered. Despite advances in the operative techniques and better understanding of the pathogenesis of wound infection, postoperative surgical site infection continues to be a major source of morbidity and mortality for patients undergoing operative procedures. Its rate varies in different countries, different areas and even in different hospitals (Ercole et al., 2007). The present study was aimed to determine the spectrum of bacterial isolates and their susceptibility patterns causing SSIs at Holy Family Hospital, Rawalpindi.
Table 3: Percent antibiotic susceptibility pattern for Gram negative bacilli isolated from SSIs
|
Antibiotics |
Bacterial isolates |
Sensitivity (%) |
|||
|
Escherachia coli (n=31) |
Pseudomonas aeruginosa (n=20) |
Provedencia spp (n=3) |
Klebsiella spp (n=3) |
||
|
Augmentin |
14(45%) |
- |
1(33%) |
2(67%) |
17(48%) |
|
Meropenem |
24(77%) |
8(40%) |
2(67%) |
3(100%) |
37(71%) |
|
Ceftazidime |
12(39%) |
- |
1(33%) |
2(67%) |
15(46%) |
|
Cefotaxime |
15(48%) |
- |
1(33%) |
1(33%) |
17(38%) |
|
Ciprofloxacin |
16(51%) |
12(60%) |
3(100%) |
1(33%) |
32(61%) |
|
Co-Trimoxazole |
11(35%) |
14(70%) |
1(33%) |
2(67%) |
28(51%) |
|
Sulzone |
17(55%) |
12(60%) |
2(67%) |
2(67%) |
33(62%) |
|
Levofloxacin |
10(32%) |
5(25%) |
1(33%) |
2(67%) |
28(39%) |
|
Colistin |
- |
15(75%) |
- |
- |
15(75%) |
|
Tetracyclin |
- |
7(35%) |
- |
- |
7(35%) |
|
Amikacin |
- |
9(45%) |
- |
- |
9(45%) |
In the present study, an overall SSIs rate of 60% was encountered which is considerably higher than those reported in the previous literature studying a number of cases not so close to our number. This relative increase in SSIs may be explained by the fact that our hospital is a teaching institution to which complex surgical cases are referred. Patients in the age group 40-60 years were infected more than those in the younger age groups. The incidence of wound infection was more in male patients (65%) as compared to female patients (35%).
In the present study, it was observed that incidence of most SSI is higher in those surgeries performed by consultants than by the postgraduate trainees and house officers. Most surgeries in this study were from abdominal region 66% followed by limbs 20% and least from chest region 2% which was also confirmed by the study done by (Petrosillo et al., 2008) who recorded highest SSI incidence in colon surgery (18.9%), gastric surgery (13.6%) and appendectomy (8.6%) operations than other non-abdominal operations.
The present study revealed that most frequently isolated organism was E. coli (40.78%) in contrast to the Nosocomial infection national surveillance service (NINSS) survey (1997–2001) which reported Staphylococcus species (47%) including S. aureus (MRSA) and S. epidermidis (Coagulase Negative) as the most common organism causing SSI (Emori et al., 1991). The results of a study conducted at Liaquat University of Medical & Health Sciences, Jamshoro, Pakistan also indicated that the frequently isolated organism was E. coli (60.7%) (Ali et al., 2009). The other potent organisms isolated were P. aeruginosa, and S. aureus which also contributes to a majority of SSI. The less common organisms were Klebsiella spp., Provedencia spp., methicillin-resistant S. aureus, S. epidermidis and MRSE.
Gram negative organisms were in access 57 (75%) as compared to Gram positive ones 19 (25%) which was also confirmed by preceding study of Pakistan (Qamar et al., 2010). While considering sensitivity patterns, all strain of pathogenic E. coli and Klebsiella spp. showed maximum sensitivity to Carbapenems (Meropenem) followed by Quinolones (Ciprofloxacin and Levofloxacin). We found S. aureus showed maximum sensitivity to Penicillin (Ampicillin).
The results of this study highlights the significance of gram negative bacilli sensitive to carbapenems and flouroquilones and for gram positive cocci against penicillin and cephalosporin’s. These antimicrobials are considered appropriate for empirical treatment of wound infections in the study area. Medical equipment, environmental surfaces, air and hands of health personnel were found to be contaminated with various types of bacterial pathogens of nosocomial importance. All professionals should take an active role in infection control within their organization and more resources should be provided to encourage good antibiotic practice and good hygiene in the hospital settings is recommended.
ACKNOWLEDGEMENTS
The corresponding author acknowledges the support and technical advises of Prof Dr. Naeem Akhtar, Rawalpindi Medical College, Rawalpindi and her parents.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest regarding this study.
Auhtor’s Contribution
Waqas Latif contributed in analysis and interpretation of data. Rao Waqas Akhtar contributed in drafting the article and revising it critically for important intellectual content. Amaila Qaisar gave her final approval of the version to be published.
REFRENCES