Journal of Infection and Molecular Biology
Review Article
Multifunctional DRB3, a MHC Class II Gene, as a Useful Biomarker in Small Ruminants: A Review
Hira Paracha1, Tanveer Hussain2, Muhammad Zahid Tahir3, Atiya Yasmeen2, Muhammad Tariq Pervez1, Ali Ahmad Sheikh4, Abbas Haider5, Rizwan Ali2, Waqas Ahmad Khan6
1Institute of Biochemistry and Biotechnology, University of Veterinary and Animal Sciences, Lahore, 54000; 2Virtual University of Pakistan, Lahore; 3Department of Theriogenology, University of Veterinary and Animal Sciences, Lahore, 54000; 4University Diagnostic Lab, University of Veterinary and Animal Sciences, Lahore, 54000, Pakistan.; 5Livestock and Dairy Development Department, Punjab, Pakistan; 6University of Sargodha, Pakistan.
Abstract | Major histocompatibility complex (MHC) is a large family of genes present in various vertebrates and is well known for the diversity of its alleles. These genes play an important role in the recognition of foreign antigens and mediating an immune response. MHC is classified as Class I, II and III molecules based on their molecular weights as well as differences in their cellular distribution and function. DRB3 gene is a member of MHC Class II and is known to show extensive polymorphism. This gene is being used as marker in phylogenetic and molecular genetics. This paper is aimed to review the role of DRB3 gene in goats.
Keywords | MHC, DRB3, Polymorphism, Immune response, Ruminants to maintain a sustainable dairy industry in the future
Editor | Tahir Yaqub, University of Veterinary and Animal Sciences, Lahore, Pakistan.
Received | October 08, 2014; Revised | November 05, 2014; Accepted | November 07, 2014; Published | January 10, 2015
Contribution | Hira Paracha and Tanveer Hussain contributed equally as first author.
*Correspondence | Tanveer Hussain, Virtual University of Pakistan, Lahore; Email: tanveer.hussain@vu.edu.pk
Citation | Paracha H, Hussain T, Tahir MZ, Yasmeen A, Pervez MT, Sheikh AA, Haider A, Ali R, Khan WA (2015). Multifunctional DRB3, a MHC class II gene, as a useful biomarker in small ruminants: a review. J. Inf. Mol. Biol. 3 (1): 19-23.
DOI | http://dx.doi.org/10.14737/journal.jimb/2015/3.1.19.23
ISSN (Online) | 2307-5465; ISSN (Print) | 2307-5716
Copyright © 2015 Paracha et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Pathogens are one of the major extermination agents reported to date (Radwan et al., 2010). Meanwhile, the decrease in genetic diversity among populations makes them more vulnerable to pathogen attack. This argument relates to the polymorphic genes of major histocompatibility complex (MHC) which initiate the adaptive immune response by encoding proteins and presents pathogen derived antigens to T cells. Although some other polymorphic genes also show effective response against pathogenic assaults but MHC genes play a primary role in immune response as compared to other polymorphic genes (Radwan et al., 2010).
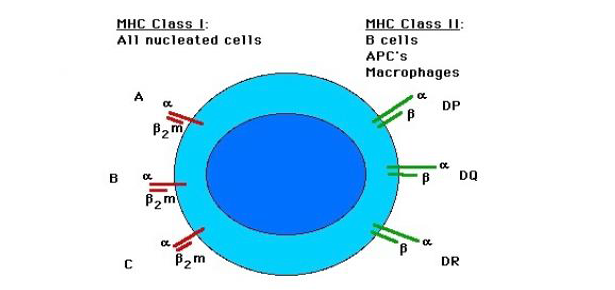
According to the Red Queen Hypothesis, the immune defense of an organism have to face the evolving pathogens constantly, hence the genetic diversity was developed in MHC in order to cope with this race for domination (Gowane et al., 2013). MHC is a group of closely linked genes which forms an important genetic component of the mammalian immune system. This group of cell surface glycoproteins plays an important role in the recognition of foreign peptide antigens (Radwan et al., 2010). MHC is classified into three main classes as I, II and III based on differences in their functions. The general structure of MHC is conserved in mammalian species although some regions in mammals are more conserved and some are less (Sheikhmohammadi et al., 2010). MHC Class I molecules are found on almost all cells and exhibit proteins to cytotoxic T cells while molecules of MHC Class II reside on specific immune cells, particularly on some antigen presenting cells (APCs) like macrophages and B cells (Figure 1)(Dukkipati et al., 2006).
STRUCTURE AND FUNCTION OF DRB3 GENE
Molecules of Class II are heterodimer glycoproteins consisting of two polypeptide chains (α and β). Under the DR region of the MHC complex, highly polymorphic β chain encoded by DRB genes is found (Zhao et al., 2011). DRA gene encodes α chain whereas β chain is coded by DRB gene (Naskar et al., 2012). Three BoLA (bovine leukocyte antigen) DRB genes (DRB1, 2 and 3) are reported in bovines but only DRB3 is functional (Singh et al., 2012). DRB1 is a pseudogene, DRB2 is expressed at lower level and DRB3 gene is highly expressed and polymorphic (Wei et al., 2010). DRB3 being a member of MHC class II genes and being polymorphic, plays significant role in disease resistance and in immune responsiveness variability (Radwan et al., 2010).
POLYMORPHIC NATURE OF DRB3 GENE
There are a large number of MHC genes and their alleles are present in most of the vertebrates. Class I play role in the recognition and binding of intracellular antigens while Class II genes are analogous to extracellular antigens (Singh et al., 2012). In higher vertebrates these polymorphic genes are grouped together and these have important role in lymphocyte mediated immune surveillance (Wei et al., 2010). Class II genes are highly polymorphic and this polymorphism is due to the large number of amino acids among alleles at each locus (Yakubu et al., 2013). The defective expression of Class II has been characterized as a disease of severe immunodeficiency called bare lymphocyte syndrome (Li et al., 2006). DRB3 is the most polymorphic class II gene in cattle and closely linked with DQ, hence DRB3 diversity is considered the diversity of entire class IIa. This polymorphism of MHC Class I and II genes is of great interest for evolutionary biologists and this genetic polymorphism is reported in various vertebrate species (Radwan et al., 2010).
SUBTYPES DRA AND DRB IN MHC RESTRICTED IMMUNE RESPONSE
In the literature, not much has been reported on caprine MHC Class II. The bovine DR and DQ are two antigens of MHC Class II and are similar to the MHC of goats named Caprine lymphocyte antigen (CLA) or goat lymphocyte antigen (GOLA). Principle Class II proteins are present on the surface of goat cells and MHC molecules of subtype DR have been recognized as member of this class. At present, two DRB loci have been identified (Radwan et al., 2007). In sheep and cattle, the MHC II genes have been largely identified while in goats only DRA and DRB genes have been sequenced. It is cleared from cytogenetic and physical mapping that Caprine MHC resides on chromosome 23 and its structural organization is same as of ovine and bovine orthologous regions (Baghizadeh et al., 2009). Among various types of MHC class II, the DQ and DR subtypes are polymorphic in human and other domestic species. These subtypes probably play a major role in the development of MHC restricted immune responses. It has been reported that there are 22 different alleles for one DRB gene in goat, but some experimental data demonstrates that there exists a second Caprine DRB locus (Amills et al., 2004). The MHC has been related to a wide variety of production traits in domestic animals. It has been examined for fertility, growth, milk and milk fat yields in cattle, chickens and swine where these factors are associated with the MHC and have developed some enhanced economic benefits. There are many genes in MHC which have a huge variety of functions but are not related to the immune response (Amills et al., 1996).
DRB3 GENE AS AN INFORMATIVE MARKER
The proteins that are important for the functioning of immune system are encoded by MHC (Othman and Ahmad, 2010). DRB locus is the most polymorphic among the MHC genes (Maillard et al., 1996). The DRB3 locus exists in the antigen presenting site and any variability in this region may lead to variability in the immune responsiveness of different individuals to particular pathogens. Due to this reason, the importance of the study of polymorphism of this locus has increased (Bot et al., 2004). The MHC has been acknowledged to regulate the progression of many infectious diseases, so it is implied that development of markers for these loci may prove helpful in pinpointing superior haplotypes for disease resistance on the condition that association between the trait and these markers can be established. The upstream regulatory region (URR) of the DRB3 gene lies approximately 200 bp upstream of the transcriptional start site and it has strong promoter/enhancer activity. This URR of the DRB3 gene consists of a series of sequence motifs like W, X, Y, CCAAT and TATA boxes. Since these motifs are highly conserved to all MHC Class II genes, their positions as well as spacing are crucial for precise transcription of BoLA genes (Behl et al., 2012). DRB3 exon 2 is highly polymorphic with >100 identified alleles (Kumar et al., 2011) and encode the antigen recognition site of the DR (Schwab et al., 2009). The ability of DRB3 to exhibit high polymorphism makes it a strong candidate to be used as a marker in molecular genetics and phylogenetic studies (Untalan et al., 2007).
DISCUSSION
The polymorphism of MHC occurs at the residues that are involved in binding of peptides and this polymorphism is maintained by some balanced selection. This variability of MHC will be helpful for evolutionary biologists and this diversity level will be expected to connect survival and welfare of a population (Bao et al., 2012). There are various forces that result the population of a gene pool. Among these forces evolutionary processes caused by adaptation to environmental factors are of great importance. In the most polymorphic regions such as MHC, the influence of these forces can be analysed by using markers. MHC genes are variable in vertebrates and play a significant role in immune response and antigen presentation (Takeshima et al., 2003). Nowadays certain advances have been made to improve animal stocks through selective breeding. The previous techniques of selection rely on subjective assessment of phenotype and progeny testing programs. These are time consuming and laborious. New techniques are being developed for the identification and isolation of DNA markers which are associated with the genes of disease resistance and economically important production traits. These markers will provide an objective system to animal breeders for the identification at the time of birth or even earlier, animals carrying desired genes (Bozkaya et al., 2007). Genetic variation is associated with resistance to pathogens. There is a chance to assess genetic variation associated with adaptive selection directly by studying genetic markers within MHC (Gogolin-Ewens et al., 1990). Due to the depletion of variation and inbreeding depression in the immunity genes makes populations more endangered to pathogens. In case of conservation efforts, variations in MHC molecules are significant. MHC presents pathogen derived antigens to the effector cells and hence they activate adaptive immune response (Castillo et al., 2010). Disease can be controlled by applying different approaches either to identify chromosomal regions or by identifying those genes that show response to vaccination. Identification of polymorphism requires large number of animals with associated genotypes and phenotypes. So, there is a requirement of certain genetic approaches such as whole genome scans using markers for the investigation of genes under complex traits or candidate genes derived from knowledge related to cellular pathways leading to pathology (Glass et al., 2012). It would be interesting to improve disease resistance by genetic means in livestock production. To select immunological marker traits is an efficient tool for the improvement of disease resistance (Eide et al., 1991).
FUTURE PROSPECTS
DRB3 gene, as a biomarker, could be used for gene assisted selection (GAS) or marker assisted selection (MAS) for the selection of safety traits and product quality. Moreover, MHC polymorphism could be used as a beneficial aid in the recognition of various pathogens.
REFERENCES