Advances in Pharmaceutical and Ethnomedicines
Research Article
Hepatoprotective Effects of Olive Oil with Fig and Date-Palm Fruit Extracts in Rats Treated with Doxorubicin and Gamma Radiation
Abdallah H. Fathy1, Mohamed A. Bashandy1, Ahmed M. Mansour2, Khaled Sh. Azab3, Samir AE. Bashandy4
1Department of Zoology, Faculty of Science, Al-Azhar University, Cairo, Egypt; 2Department of Pharmacology and Toxicology, Faculty of Pharmacy, Al-Azhar University, Cairo, Egypt; 3Department of Radiation Biology, National Center for Radiation Research and Technology, Atomic Energy Authority, Cairo, Egypt; 4Department of Pharmacology, National Research Center, Cairo, Egypt.
Abstract | Chemotherapy and radiotherapy are among the traditional methods used in cancer treatment and they cause toxicity to normal cells. The purpose of the present study is to determine the possible hepatoprotective role of olive oil (7 g/kg) with fig (1 g/kg) and date-palm fruit (1 g/kg) extracts (OFD) together as potent antioxidants in alleviating the toxicity of doxorubicin (DOX) and/or gamma radiation in albino Wistar rats. We injected the rats with DOX (2.5 mg/kg, i.v.) and/or exposed the rats to irradiation (2 Gy, whole body) weekly, for four consecutive weeks. We used the antioxidant treatments daily via oral gavages for two weeks protection period and during the experiment (4 weeks). The DOX and/or irradiated groups recorded severe depletion of antioxidant parameters (superoxide dismutase, catalase and reduced glutathione) as well as increased thiobarbituric acid reactive substances, advanced oxidation protein products, and alterations of liver function parameters (transaminases, alkaline phosphatase, gamma glutamyl transferase, total protein, albumin and bilirubin) as compared with control rats. Administration of OFD to DOX and/or irradiated rats significantly ameliorated the oxidative stress markers and liver function parameters as compared with DOX and/or irradiated rats. In conclusion, the olive oil with fig and date-palm fruit extracts together could be used synergistically to decrease the bad side effects of chemotherapy and radiotherapy.
Keywords | Olive oil, Fig fruit extract, Date-palm fruit extract, Doxorubicin, Gamma radiation, Oxidative stress.
Editor | Mayada Ragab Farag, Forensic Medicine and Toxicology Department, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt.
Received | September 07, 2017; Accepted | October 17, 2017; Published | November 08, 2017
*Correspondence | Abdallah Hussien Fathy, Zoology Department, Faculty of Science, Al-Azhar University, Nasr City, Cairo, Egypt; Email: abdallah.ahf@gmail.com, abdallahhfathy@pharma.asu.edu.eg
Citation | Fathy AH, Bashandy MA, Mansour AM, Azab KS, Bashandy SA (2017). Hepatoprotective effects of olive oil with fig and date-palm fruit extracts in rats treated with doxorubicin and gamma radiation. Adv. Pharm. Ethnomed. 5(1): 8-15.
DOI | http://dx.doi.org/10.17582/journal.ape/2017/5.1.8.15
ISSN | 2310-0575
Copyright © 2017 Fathy et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
The reactive oxygen species (ROS) are highly active molecules containing oxygen that reacts with biological molecules and destroy the cells (Baatout et al., 2004). The increased ROS formation and decreased antioxidant protection caused oxidative stress resulting in cell death (Turrens, 2003). In addition, they are responsible for protein denaturation and impaired enzyme activity (Karbowink and Reiter, 2000). Doxorubicin is a potent antibiotic used for the treatment of different tumors (Patil et al., 2008). The rats injected with doxorubicin and/or exposed to ionizing radiation induced oxidative stress and several side effects as well as irreversible toxicity (Maity et al., 1994; Elsadek et al., 2017).
The major benefit of the Mediterranean diet including olive oil, figs and date palm is its high level of natural antioxidants, which contribute antioxidant vitamins, minerals and phenolic compounds (Solomon et al., 2006). Plant extracts showed a higher diversity of active ingredients resulted in greater stability and bioaccessibility of antioxidants (Bashandy et al., 2014; Kamiloglu et al., 2014). Some researchers claimed that the use of chronic administration of mixed plant extracts might augment the major cellular endogenous antioxidants, and so it could be identified as a promising strategy to combat the oxidative stress (Lemos et al., 2012; Bashandy et al., 2014; Bashandy et al., 2016).
In light of the previous hypothesis, the present study aims to investigate the potential hepatoprotective effects of olive oil with fig and date-palm fruit extracts together, as potent antioxidants, in their recommended antioxidant doses, synergistically, against doxorubicin and gamma radiation induced oxidative stress in Wistar rats.
Materials and methods
Doxorubicin (DOX)
We purchased doxorubicin from EIMC united pharmaceuticals, Co. Egypt as Adricin® (doxorubicin hydrochloride). The doxorubicin injection dose in the present study was 2.5 mg/kg, i.v., weekly for four consecutive weeks (10 mg/kg body weight cumulative doses).
Irradiation (R)
We used the Canadian Gamma cell–40 (137Cs) (National Center for Radiation Research and Technology (NCRRT), Nasr City, Egypt) to irradiated rats with a whole body fractioned dose (2 Gy every week for four weeks up to 8 Gy cumulative doses). The dose rate at the time of the experiment was 0.45 Gy/min (4.44 min exposure time).
Plant Materials
We purchased the extra virgin olive oil (Olea europaea L.; Family Oleaceae) from The Grup Pons Company (Spain). The dried fruits of fig (Ficus carica L. Family: Moraceae) were procured from Kafoods Ltd. (Turkey). The fruits of date palm (Phoenix dactylifera L., Family: Arecaceae) were purchased from Al-MADINA AL-MUBARAKA market, Saudi Arabia.
The expert taxonomist Dr. Al-Baraa El-Saied (Department of Botany, Faculty of Science, Al-Azhar University, Cairo, Egypt) identified and authenticated the plant materials. The voucher specimens of the authenticated plant materials were deposited at the medicinal plants research station (MPST), Faculty of Pharmacy, Ain-Shams University, Cairo. Egypt.
Plant Extracts
Fig fruit extract (Ficus carica L.; Moraceae): The fruits were cut into small pieces and dried in an oven at 40 °C. The dried pieces were coarsely grounded by an electrical device. The powdered material was mixed with five folds of aqueous ethanol (80%) for 72 h with occasional shaking. The soaked material was rendered free from plant debris by filtering through a fine filter paper and subjected to evaporation under reduced pressure on a rotary evaporator. Then, the crude extract was further dried, lyophilized, collected and stored at -20 °C until used (Gilani et al., 2008).
Date palm fruit extract (Phoenix dactylifera L; Arecaceae): The date fruits were rendered free from soil and adulterated materials, separated from pits, cut into pieces, dried in an oven and coarsely ground by an electrical device. The ethanol extract was done by using a mixture of aqueous ethanol (50%) and pounded date fruits (3:1, volume to weight) for 48 h in a refrigerator with continuous stirring (Al-Qarawi et al., 2005). The whole solution was ground, then centrifuged at 4 °C for 20 min at 1788g. The supernatant was collected, dried, lyophylized and stored at -20 °C until use (Vayalil, 2002).
Ethics Statement
The present study was conducted in accordance with the ethical guidelines for investigations of laboratory animals (Institute of Laboratory Animal Resources, 1996) and approved by an independent ethics committee of the National Centre for Radiation Research and Technology.
Experimental Animals
The present study used 120 male Wistar albino rats (150-170 g) obtained from the Egyptian Holding Company for Biological Products and Vaccines (VACSERA, Cairo, Egypt). Rats were maintained at the animal center, Faculty of Pharmacy, Al-Azhar University, Cairo, Egypt. They were kept in a temperature-controlled environment (20-25 °C) and 50%–60% relative humidity with an alternating 12 h light-dark cycle. After 2 week acclimatization, we divided the rats into 8 groups (Table 1). Five rats were placed into each cage and provided with standard diet pellets and drinking tap water ad libitum during the whole experimental period.
The Antioxidant Doses
The selected antioxidant doses were olive oil 7.6 ml/kg (7 g/kg), fig fruit extract 1 g/kg & date-palm fruit extract 1 g/kg body weight by oral gavages for six consecutive weeks. The selection of doses was based on the human recommended antioxidant doses of extra virgin olive oil (Bashandy et al., 2014), dry figs and date palm (Vinson et al., 2005) after conversion to rat doses (Reagan-Shaw et al., 2008). The FDA recommended standard serving size of dried fruits for human is 40 g. In the present study, the serving size of figs was equivalent to 10 g extract (about 3 figs) and the serving size of dates was equivalent to 10 g extract (the flesh of 7 dates).
Collection and Preparation of Samples
We collected blood samples at the end of the experiment from each animal under anesthesia from the retro-orbital plexus using capillary tubes. Blood samples were collected and put into non-heparinized plain tubes, which were centrifuged at 1788g for 10 min. The serum samples were frozen at -80 °C for the following measurements. After sampling, animals were sacrificed and livers were isolated, dissected out and washed with isotonic saline. Each tissue was homogenized in ice-cold Tris–HCl lysis buffer, pH 7.4 containing 1% protease inhibitor. The homogenates were centrifuged under cooling at 1006g for 20 min. The supernatants were subsequently aliquoted and stored at -80 °C until used.
Table 1: Study design of the protective effects of olive oil with fig and date-palm fruit extracts (OFD) in rats treated with doxorubicin (DOX) and/or gamma radiation (R).
| Groups | No. of rats |
| 1- Control | 15 |
| 2- OFD | 15 |
|
3- DOX |
15 |
| 4- R | 15 |
| 5- DOX-R | 15 |
| 6- OFD-DOX | 15 |
| 7- OFD-R | 15 |
| 8- OFD-DOX-R | 15 |
| Sum | 120 |
OFD: Olive oil: 7.6 ml/kg (7 g/kg), Fig fruit extract: 1 g/kg & Date-palm fruit extract: 1 g/kg body weight by oral gavages for six consecutive weeks; DOX: Doxorubicin injection (2.5 mg/kg, i.v.), weekly for four consecutive weeks; R: Whole body γ-irradiation, 2 Gy weekly for four consecutive weeks; DOX-R: Rats exposed to irradiation following 20 h of DOX injection as the same schedules mentioned above.
Biochemical Study
The liver tissue homogenates were used for the determination of hepatic thiobarbituric acid reactive substances (TBARS) (Yoshioka et al., 1979), hepatic advanced oxidation protein products (AOPPs) (Witko-Sarsat et al., 1996) and hepatic reduced glutathione (GSH) (Beutler et al., 1963). In addition, the activities of superoxide dismutase (SOD) (Minami and Yoshikawa, 1979) and catalase (CAT) (Aebi, 1984) enzymes were estimated in the homogenates using kits from bio-diagnostic Co., Egypt.
The serum levels of transaminases (AST & ALT) (Bergmeyer et al., 1986), alkaline phosphatase (ALP) (German Society for Clinical Chemistry, 1972), total protein (TP) (Gornal et al., 1949), albumin (Doumas et al., 1971), gamma glutamyl transferase (GGT) (Szasz, 1969) and total bilirubin (TBIL) (Scherwin and Thompson, 2003) were estimated using kits from Elitech diagnostic Co., France.
Statistical analysis
We used statistical package for social sciences SPSS/PC computer program (version 19, USA) for the statistical analysis of the present results. The results were analyzed using analysis of variance (ANOVA) one-way test followed by least significant difference test for multiple comparisons. Differences were considered statistically significant at p<0.05. Data are summarized as mean ± standard error.
Results
Oxidative Stress Markers
The DOX and/or irradiated groups for four weeks recorded a significant elevation (p<0.05) in the hepatic thiobarbituric acid reactive substances (TBARS) and hepatic advanced oxidation protein products (AOPPs) as compared with the corresponding values in the control group. In addition, a significant decrease (p<0.05) in hepatic reduced glutathione (GSH), superoxide dismutase (SOD) & catalase (CAT) levels were recorded at the DOX and/or irradiated groups as compared with the corresponding values in the control group (Table 2 and Figure 1).
Table 2: The protective effects of olive oil with fig and date-palm fruit extracts on the oxidative stress markers in the liver tissue of different groups.
|
Parameters Groups |
TBARS |
AOPPs |
SOD |
CAT |
| Control |
196±1.52a |
196±0.99a |
74.33±0.87a |
1.54±0.01a |
| OFD |
194±1.71a |
194±0.73a |
75.14±1.43a |
1.55±0.01a |
| DOX |
224±1.59b |
222±0.78b |
65.17±1.69b |
1.25±0.01b |
| R |
221±1.59b |
219±1.19c |
66.04±1.83b |
1.34±0.01c |
| DOX-R |
238±1.50c |
232±1.92d |
60.35±1.45c |
1.06±0.01d |
| OFD-DOX |
214±2.58d |
210±1.89e |
70.20±1.63d |
1.45±0.01e |
| OFD-R |
213±2.66d |
208±1.98e |
70.29±1.24d |
1.49±0.00f |
| OFD-DOX-R |
231±1.69e |
228±1.24f |
64.61±1.22b |
1.16±0.01g |
Note: Results are expressed as mean ± standard error. For each parameter, values not sharing common superscript letters (a-g) are significant with each other at p<0.05; OFD, olive oil with fig & date palm; DOX, doxorubicin, R, irradiation; TBARS, thiobarbituric acid reactive substances; AOPPs, advanced oxidation protein products; SOD, superoxide dismutase; CAT, catalase; GSH, reduced glutathione.
The DOX and/or irradiated groups received two weeks pretreatment with OFD and during the experimental period (four weeks) showed a significant decrease (p<0.05) in the hepatic TBARS, AOPPs and a significant increase
(p<0.05) in the hepatic GSH, SOD & CAT as compared with the DOX and/or R groups not treated by OFD, respectively (Table 2 and Figure 1).
Table 3: The protective effects of olive oil with fig and date-palm fruit extracts on the serum liver function parameters of different groups.
| Parameters Groups | AST (U/L) | ALT (U/L) | TP (g/dl) | ALB (g/dl) | GLOB (g/dl) | A/G ratio (g/dl) |
| Control |
71.97±1.22a |
65.00±1.62a |
6.23±0.07a |
4.04±0.06a |
2.19±0.09a |
1.88±0.10a |
| OFD |
71.34±1.70a |
64.22±1.85a |
6.29±0.06a |
4.14±0.04a |
2.15±0.08a |
1.94±0.09a |
| DOX |
98.81±2.68b |
118.0±3.02b |
5.53±0.04b |
2.83±0.06b |
2.69±0.06b |
1.06±0.05b |
| R |
94.34±2.69c |
103.0±2.78c |
5.77±0.02c |
3.04±0.05c |
2.73±0.06b |
1.12±0.04bc |
| DOX-R |
112.7±2.15d |
134.5±2.21d |
5.19±0.05d |
2.26±0.04d |
2.93±0.08c |
0.77±0.03d |
| OFD-DOX |
81.99±1.66e |
80.68±2.60e |
5.90±0.05c |
3.32±0.04e |
2.58±0.05bd |
1.28±0.03ef |
| OFD-R |
77.39±1.66f |
73.85±2.38f |
6.08±0.08e |
3.58±0.02f |
2.50±0.08de |
1.44±0.05e |
| OFD-DOX-R |
85.57±1.36e |
87.06±3.18g |
5.28±0.07d |
2.89±0.05b |
2.39±0.10e |
1.22±0.07cf |
Note: Results are expressed as mean ± standard error. For each parameter, values not sharing common superscript letters (a-g) are significant with each other at p<0.05; OFD, olive oil with fig and date palm; DOX, doxorubicin; R, irradiation; AST, aspartate aminotransferase; ALT, alanine aminotransferase; TP, total protein; ALB, albumin; GLOB, globulin; A/G, albumin-globulin ratio.
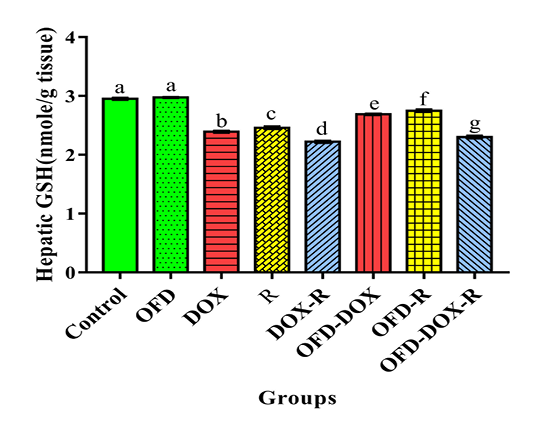
Figure 1:The protective effects of olive oil with fig & date palm extracts (OFD) on hepatic reduced glutathione (GSH) of doxorubicin (DOX) and/or gamma irradiated (R) groups. Columns not sharing common superscript letters are significant with each other at p<0.05.
Liver Function Parameters
The DOX and/or irradiated groups recorded a significant elevation (p<0.05) in serum transaminases (AST & ALT), alkaline phosphatase (ALP), gamma glutamyl transferase (GGT), total bilirubin (TBIL) & globulin and a significant decrease (p<0.05) in serum total protein (TP), albumin & A/G ratio as compared with the corresponding values in the control group (Table 3 and Figure 2, 3).
The DOX and/or irradiated groups received two weeks pretreatment by OFD and during the experimental period (four weeks) showed a significant decrease (p<0.05) in the serum AST, ALT, ALP, GGT, TBIL & globulin and a sig-
nificant increase (p<0.05) in the serum TP, albumin & A/G ratio as compared with the DOX and/or R groups not treated by OFD, respectively (Table 3 and Figure 2, 3).
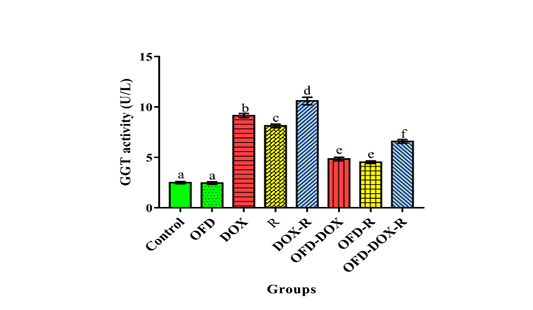
Figure 2: The protective effects of olive oil with fig & date palm extracts (OFD) on serum gamma glutamyl transferase (GGT) activity of doxorubicin (DOX) and/or gamma irradiated (R) groups. Columns not sharing common superscript letters are significant with each other at p<0.05.
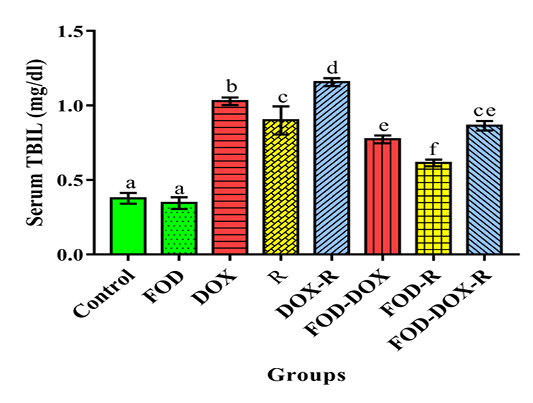
Figure 3: The protective effects of olive oil with fig & date palm extracts (OFD) on serum total bilirubin (TBIL) of doxorubicin (DOX) and/or gamma irradiated (R) groups. Columns not sharing common superscript letters are significant with each other at p<0.05.
Discussion
The DOX treatment and/or radiation exposure creates DNA damage, cytotoxicity and morphological changes causing oxidative stress and generation of ROS (Fang et al., 2002). The increased formation of TBARS and AOPPs in response to DOX and/or radiation exposure in the present study has been associated with an early marker of oxidative stress because they are correlated well with increased production of ROS (Baskol et al., 2006). In addition, the present results fulfilled well with Witko-Sarsat et al. (1996) who suggested that AOPPs accumulation coexisted with decreased GSH level support the occurrence of oxidative stress.
The endogenous antioxidant system contains several natural enzymes (SOD & CAT) and non-enzyme (GSH) defenses (Halliwell et al., 1992). The DOX injection and/or exposure to ionizing radiation leads to depletion of these endogenous antioxidants (Adem et al., 2014). The SOD is the only enzyme that utilizes the free radicals as a substrate and catalyzes the reduction of superoxide radical to hydrogen peroxide (Balin and Allen, 1986). The significant decrease in SOD activity in the liver tissue of DOX and/or irradiated rats might be due to the overproduction of free radical. The enzyme is one of the self-defense mechanisms against oxidative stress where it constitutes the first line of defense against the deleterious effects of ROS (Guo et al., 2003).
CAT is an inducible enzyme, decomposes hydrogen peroxide, and is involved in the antioxidant defense mechanisms of mammalian cells. Thus, it is an index of increased hydrogen peroxide (Meneghini, 1997). The catalase enzyme converts hydrogen peroxide to water and oxygen (Balin and Allen, 1986). The present study showed a significant decrease in the activity of CAT recorded in the liver tissue of DOX and/or irradiated rats as compared with the control rats. This decrease might be due to the oxidative modification of various protein types, leading to functional alterations and physiological impact (Prasad et al., 2005).
The present results showed a significant decrease in GSH concentration in the liver tissue of DOX and/or irradiated rats as compared with the control rats. The GSH deficiency contributes to oxidative stress (Wu et al., 2004). In addition, the depletion in GSH level after DOX and/or radiation exposure may be due to its diffusion through impaired cellular membranes or inhibition of GSH synthetase and glutathione reductase enzymes (Zahran et al., 2006). In the same concern, Srinivasan et al. (2007) showed that the decreased levels of GSH might be due to its utilization by the enhanced production of ROS.
The oxidative stress markers in the liver of DOX and/or irradiated rats in the present study showed increased TBARS and AOPPs, and decreased SOD, CAT and GSH activities. The alterations in oxidative stress markers may be due to the radio-sensitizing effect that might be probably related to the inability of cells to cope with the overproduction of different free radicals (Weiss and Landauer, 2003).
The clinical and diagnostic values associated with increased blood enzyme concentrations of AST, ALT, ALP and GGT as well as TBIL have long been recognized (Martin and Freidman, 1998). The elevated liver marker enzymes in the blood serum reflected the radical-mediated lipid peroxidation of the liver cell membranes. These free radicals combined with the cellular proteins, which in turn, initiate lipid peroxidation (TBARS) and protein carbonylation (AOPPs), resulting in structural changes of bio-membranes and loss of liver integrity and decreased metabolic activity (Güven and Gülmez, 2003). The increased levels of these diagnostic markers of hepatic function in DOX and/or irradiated rats are implicative of the degree of hepatocellular dysfunction. The increase in transaminases was the clearest indication of cellular leakage and loss of functional integrity of the membrane of liver cells (Elsadek et al., 2017; Fathy et al., 2017). In addition, the elevated liver markers (ALP, GGT and TBIL) could be attributed to bile duct obstruction or infiltrative diseases of the liver (Carl and Endword, 2001; Fathy et al., 2017).
An important function of the serum protein is the maintenance of the normal distribution of the body water by controlling the osmotic balance between the circulating blood and the cells (Harper et al., 1977). The results in the present work showed a significant decrease in serum total proteins, albumin and A/G ratio in DOX and/or irradiated groups. The decrease in serum protein might be the result of damaged biological processes or due to the changes in the membrane permeability of the liver and kidney tissues, resulting in leakage of protein, especially albumin through the kidney (Ali et al., 2007; Haggag et al., 2008).
The olive oil as well as fig and date palm (OFD) are natural antioxidants rich in phenolic compounds and many other active ingredients and used in the present study on their recommended antioxidant doses. Recently, phenolic compounds showed several biological activities such as antioxidant, anticancer and hepatoprotective activities (Bashandy et al., 2014; El Arem et al., 2014; Bashandy et al., 2016).
In light of the present results, the protective action of OFD against lipid peroxidation and protein oxidation as factors modifying membrane organization might be related to their ability to scavenge the oxidation-initiating agents (Sánchez-Moreno et al., 1998). The possible mechanism of the anti-hepatotoxic effect of OFD may be attributed to their antioxidant activity due to their phenolic and other compounds, which are able to donate a hydrogen atom to the free radicals, thus stopping the deleterious reaction processes (Bashandy et al., 2014). This effect was evidenced by their ability to ameliorate the activities of the oxidative stress markers of TBARS, AOPPs, SOD, CAT and GSH levels in the liver tissue homogenate.
In agreement with Chainy et al. (2000), the olive oil could be used to protect the liver of experimental animals from chemical injuries. Moreover, hydroxytyrosol of olive oil reported to be an effective radical scavenger (Nivitabishekam et al., 2009). The antioxidant effect of the figs might be attributed to the effective antioxidant component called Cyanidin-3-rhamnoglucoside identified to inhibit protein oxidation and reduce the oxidative stress (Solomon et al., 2010). Singh et al. (2012) reported that the hydroalcoholic extract of fruit of Ficus carica was responsible for ameliorating various biochemical and antioxidant parameters and provided hepatoprotection against toxic chemicals. In addition, the present data agreed with previous studies (El Arem et al., 2014; Ahmed et al., 2015; Bashandy et al., 2016) which showed a significant amelioration in hepatic parameters and oxidative stress markers in the liver of chemically intoxicated or irradiated rats treated with date palm extract through antioxidant mechanisms.
The mechanisms of action of most of the hepatoprotective drugs belonging to the group of free radical scavenger involve membrane stabilization, neutralization of free radicals and immunomodulation (Azab and Nada, 2004). The treatment with OFD in the present study reveals the presence of numerous and varied antioxidant agents. The diverse natural antioxidants act synergistically with each other to produce antioxidative activities against the free radical (Viola and Viola, 2009).
In conclusion, the administration of extra virgin olive oil with fig and date palm extract to DOX and/or irradiated rats ameliorated the oxidative stress markers and serum liver function parameters revealing a synergistic effect of the combination between them. Therefore, the present study suggests the combination therapy using olive oil with fig and date palm for decreasing the bad side effects of therapy in patients treated with chemotherapy and/or radiotherapy. These findings need to be further validated in human clinical trials.
Conflicts of interest
The authors declare no competing financial interest.
Authors contribution
This work was carried out in collaboration between all authors. All authors read and approved the final manuscript.
References





