Advances in Pharmaceutical and Ethnomedicines
Research Article
Investigating the Immunomodulatory Effect of Cassia fistula on Albino Rats
Vijay Laxmi1*, Nitin Wahi2, Anubhuti Sharma3, Anjana Goel2, Ashok Kumar Bhatia2
1Department of Microbiology, Pt. Deen Dayal Upadhyaya Pashu Chikitsa Vigyan Vishwavidhyalaya Evam Go Anusandhan Sansthan (DUVASU), Mathura 281001, Uttar Pradesh, India; 2Department of Biotechnology and Microbiology,2GLA University, Mathura 281006 (U.P.), India; 3Department of Biosciences & Biotechnology, Banasthali University, Jaipur 281006 (Raj.), India.
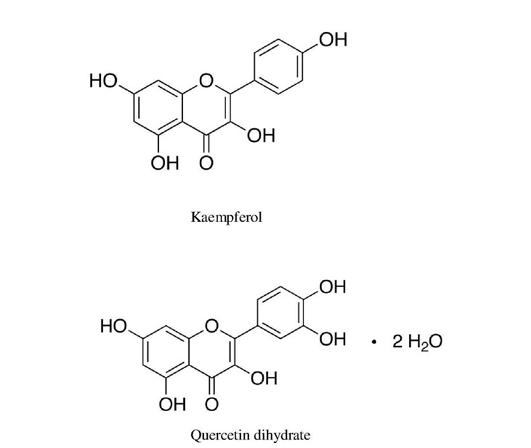
Abstract | The present study was aimed to evaluate the immunomodulatory activities of hot aqueous extract of pods & leaves (HAEP & HAEL) of C. fistula in albino rats. HAEP/HAEL were given oral dose of 125, 250 & 500 mg/kg to healthy rats divided into one unfed (control group) and six fed groups. The humoral immune response was carried out by testing antibody titer against Salmonella typhimurium ‘O’ antigen by tube agglutination test and cellular immune response was carried out on DNCB (1-Chloro-2, 4-dinitrobenzene) sensitized albino rats. Results showed that agglutination titer in all HAEP treated rats ranged from 153.6+25.6 to 256.0+62.71 and HAEL treated rats exhibited antibody titer from 358.4+62.71 to 409+51.2 in relation to control & other groups. HPLC analysis of HAEP, HAEL for determination of active immunomodulants have confirmed the presence of Quercetin dihydrate within HAEP, HAEL and Kaempferol only within HAEL. Thus the present study concludes that rise in humoral immune response may be due to the stimulation of B- lymphocytes, T-helper Cells & macrophages subsets by pods, leaf extracts containing immunomodulants Quercetin dihydrate and Kaempferol. Hence it is concluded that C. fistula plant has immunomodulatory activity, although detailed study regarding digestibility, hepatic toxicity & molecular mechanism of action are needed to be carried out.
Keywords | Cassia fistula, ‘O’ antigen, Immunomodulatory activity, Quercetin dihydrate, Kaempferol
Editor | Gulzeb Aziz, Norwegian Medicinal Agency, Oslo, Norway.
Received | August 30, 2015; Revised | September 14, 2015; Accepted | September 15, 2015; Published | October 15, 2015
*Correspondence | Vijay Laxmi, Department of Microbiology, 1DUVASU, Mathura 281001 (U.P.), India; Email: vijaylaxmitripathi82@gmail.com
Citation | Laxmi V, Wahi N, Goel A, Bhatia AK (2015). Investigating the immunomodulatory effect of Cassia fistula on Albino rats. Adv. Pharm. Ethnomed. 3(1): 1-5.
DOI | http://dx.doi.org/10.14737/journal.ape/2015/3.1.1.5
ISSN | 2310-0575
Copyright © 2015 Laxmi et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
The greatest threat to human population on Earth comes from microbes (Casadevall and Relman, 2010). These microbes are ubiquitous in our environment and have affected the development, evolution of every human civilization. To combat these ubiquitous killers and to survive in an environment hosted by pathogens a remarkable, versatile, defense system has evolved in animals called as Immune system (Delves et al., 2010). Although Immune system is an autonomous, inbuilt defense system present in all living animals, yet humans have always shielded themselves against these microbes using antibiotic (Tortora et al., 2009).
The commercialization of these antimicrobial agents by pharmaceutical companies and related economic benefits has fascinated scientists to explore new broad spectrum antibiotics (Monnet, 2005). The practice of indiscriminate use of these antibiotics have led to the development of multi drug resistant (MDR) strains of microbes, evidenced from the recent epidemic caused by influenza virus, ebola virus, methicillin resistant Staphylococcus aureus, etc. (Davies and Davies, 2010; Monnet, 2005; Vimalraj et al., 2009; Spellberg et al., 2008). The gridlocks associated with modern medicines such as several side effects, ever increasing immunological disorders, development of MDR strains have enforced researchers globally to move back to basic approach, to combat microbes with the use of medicinal plants as an immunostimulant (Vimalraj et al., 2009).
One of the most well documented plants in Vedas & Upanishads is Cassia fistula (Caesalpiniaceae) (Bahorun et al., 2005). Also known as golden shower tree, C. fistula is a native of Indian subcontinent & occupies significant position in the indigenous system of medicine of many Asian, African and South American countries (Bahorun et al., 2005; Bhalerao and Kelkar, 2012; Danish et al., 2011). In ancient ayurvedic system C. fistula has been used for the treatment of various ailments such as pruritus, leucoderma, diabetes, haematemesis, etc (Danish et al., 2011; Rizvi et al., 2009; Seyyednejad et al., 2014; Yadav and Jain, 1999). Besides C. fistula, other species of cassia such as C. alata, C. sophera, C. javanica, C. auriculata plants have also been studied and are claimed to be of therapeutic importance (Abo et al., 2000; Bhalerao and Kelkar, 2012; Bhuvaneswari and Gobalakrishnan, 2014). Although the plant has a high therapeutic value and reported to possess hepatoprotective, anti-inflammatory, antitussive, antifertility, antimicrobial and antitumor activities within itself, yet little information is available regarding its role in immunomodulation. Thus the present study was taken up to investigate out the folklore use of the plant, determining immunomodulatory property of its aqueous pod & leaf extract with scientific validation.
Materials and Methods
Collection of Plant Materials, Preparation of Hot Aqueous Extract of Pods & Leaves
Fresh pods and leaves of Cassia fistula were collected separately from the month of May to June from Mathura and its adjoining areas. Cassia fistula plant was identified and authenticated at the department of Botany, B.S.A. College, Dr B.R. Ambedkar University. The pod, leaves were dried in shade and coarsely powdered. The dried powder was extracted with triple distilled water using soxhlet apparatus. The extracts obtained were evaporated to dryness at 45°C using hot air oven and weighted.
Effect of HAEP and HAEL on Humoral Immune System
The humoral immune response was measured by determining the amount of antibody raised in different groups of rats fed with ‘O’ antigen and different concentration of HAEP, HAEL extract.
Preparation of Salmonella typhimurium ‘O’ antigen
Salmonella typhimurium culture was obtained from the department of Microbiology and immunology, Pandit Deen Dayal Upadhyay Pashu Chikitsa Vigyan Vishwavidylaya Evam Go Anusandhan Sansthan (DUVASU), Mathura. Prior to experimentation, the bacterial isolates were characterized on the basis of their shape, microscopic examination, gram staining, ability to ferment sugars, biochemical test such as catalase, oxidase, etc (Cruickshank et al., 1975). After confirmation S. typhimurium ‘O’ antigen was prepared by standard methods (Bhatia et al., 2003). Smooth colonies of S. typhimurium grown on Tryptose agar medium were selected and inoculated in nutrient broth. Inoculated broth was incubated for eight hours at 37°C and then centrifuged at 3000 rpm for 20 minutes, subsequently supernatant was discarded and pellet obtained was washed with normal saline and then boiled at 100°C for two hours thirty minutes. This heated culture was then used as ‘O’ antigen for determination of humoral immune response in albino rats.
Immunization of Rats
Experimental rats were divided in seven groups of albino rats with 5 rats in each group. The Ist group of rats in which only S. typhimurium ‘O’ antigen was inoculated served as the negative control, as no plant extract was provided to the rats of this group. Group IInd, IIIrd & IVth rats were fed orally, with 125 mg/kg, 250 mg/kg & 500 mg/kg of HAEP respectively for 21 days, (Weichert, 1940) while albino rats of group Vth, VIth and VIIth were also fed orally with similar concentration of HAEL respectively for 21 days along with ‘O’ antigen in all the above groups. One week after last dose of ‘O’ antigen was provided, blood serum was collected for determining antibody titer in rats against Salmonella by serum agglutination test. The presence of active immunomodulants within HAEP and HAEL was determined through HPLC analysis of both the extracts using different standards.
Effect of HAEP and HAEL on Cell Mediated Immune System
Study was conducted to access the effect of HAEP/ HAEL on DNCB (1-chloro-2,4-dinitrobenzene) sensitized albino rats (Tiwary and Goel, 1985). In this experiment DNCB acted as allergen. The experiments were conducted over seven groups of albino rats, with five rats in each group. The Group Ist rats were used as a negative control as only DNCB was provided with no plant extract. Group IInd, IIIrd & IVth rats were fed with 125 mg/kg, 250 mg/kg & 500 mg/kg of HAEP and similar concentrations of HAEL were provided to the rats of group Vth, VIth and VIIth for 21 days.
Hair were removed from thigh of rats of all groups and cleaned properly. 100 µl of 0.2% of DNCB (dissolved in acetone) was applied on hairless thigh skin of albino rats in all the groups on 7th, 14th, 17th day, respectively. Challenge dose was applied on 22nd day at the same site. Skin thickness was measured with the help of Vernier Caliper at 0, 24, 48 and 72 hrs post challenge. Difference in the skin thickness measured at 0, 24, 48 and 72 hrs was calculated. The inoculated site was also examined for erythema, induration and vesicle formulation.
Results and discussion
Induction of Humoral Immune Response
Results obtained clearly illustrated that antibody titer in the serum samples of all treated rats was higher in relation to control. Further, the result establish the fact that HAEL fed rats exhibited much higher antibody titer (358.4±22.71, 409.6±22.71, 409.8±21.2), over the serum antibody titer raised in HAEP fed group (153.6±15.6, 256.0±12.7, 256.0±12.6) and that of control (115.28 ±12.7) (Figure 1).
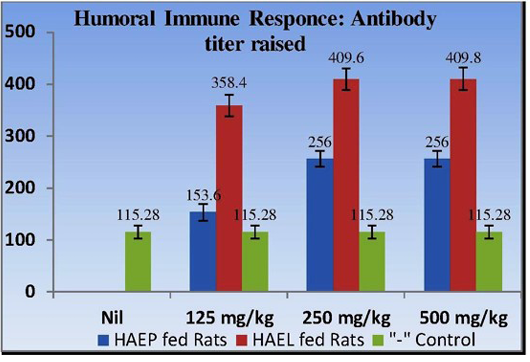
Figure 1: Determining the humoral immune response of albino rats by measuring antibody titer in rats fed with 125, 250 and 500 mg/kg of HAEP and HAEL with respect to control
This study confirms that there is a significant rise in antibody titer, thus humoral immune response is induced in HAEL and HAEP fed rats with significant higher immunomodulatory effect in HAEL fed rats. The determination of active immunomodulants within HAEP and HAEL through HPLC confirms the presence of Quercetin dihydrate among both the extracts and Kaempferol only within HAEL (Figure 2) (Laxmi et al., 2015).
The presence of Quercetin dihydrate in both extracts might be responsible for elevated humoral immune response as Quercetin has been reported to up regulate IFN-γ & Th-2 gene expression and production, which in turn modulate NK cell function and act as immunostimulant (Nair et al., 2002). Quercetin has also been shown to display anti-inflammatory and antibacterial activities (Toit et al., 2009). Further the hyper immune response in case of HAEL
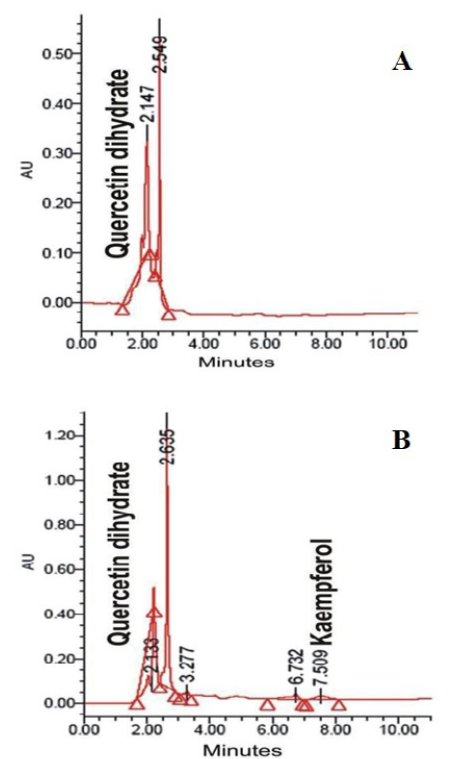
Figure 2: A) HPLC analysis of hot aq. Extract of pods (HAEP) displaying the presence of Quercetin dihydrate; B) HPLC analysis of hot aq. Extract of leaves (HAEL) displaying the presence of Quercetin dihydrate and Kaempferol
could be credited to the presence of Kaempferol which has been reported to display a significant increase in phagocytic index and has been shown to exhibit dose dependence enhancement in the production of T-Lymphocytes (CD3 and CD19), Th1 Cytokines (IL-2, IFN-γ and IL-4) (Figure 3) (Swarnalatha and Puratchikody, 2014).
Induction of Cell Mediated Immune Response
Almost all doses of both extracts exhibited a marked enhancement in skin thickness. Rats fed with 250 mg/kg of body weight HAEP displayed max increase in skin thickness by 27.1%, 21.1% & 14.5% at 24, 48 and 72 hrs,
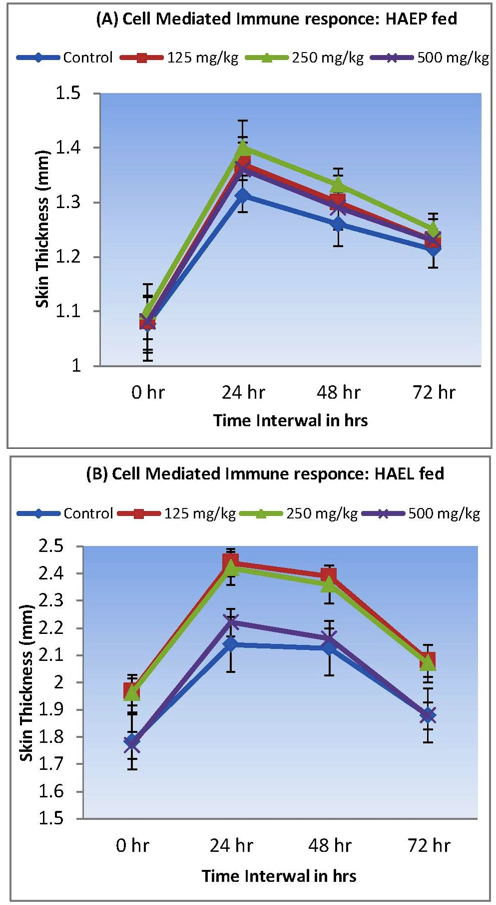
Figure 4: A)Increase in skin thickness of albino rats fed with 125, 250 and 500 mg/kg of HAEP recored at 24, 48 and 72 hrs time intervals with respect to control; B) Increase in skin thickness of albino rats fed with 125, 250 and 500 mg/kg of HAEL recored at 24, 48 and 72 hrs time intervals with respect to control
respectively (Graph-II). Study on rats fed with 125 mg/kg HAEL indicated best increase in skin thickness by 33.15%, 22.67% & 16.33% at 24, 48 and 72 hrs with respect to control (Graph-II). Results also indicated that albino rats fed with 500 mg/kg body weight of HAEP & HAEL induced less increase in their skin thickness with respect to albino rats fed with 125 mg/kg body weight of either extract. The best cell mediated immune response has been achieved in rats fed with 250 mg/kg of HAEP & 125 mg/kg of HAEL (Figure 4).
Comparative analysis with parallel studies suggest patients treated with C. fistula pulp showed a significant decrease in serum IgE level producing significant relief in patients suffering from Eczema or Leprosy (Das et al., 2008). Similarly several researchers have demonstrated the immune system modulation activities of C. auriculata & C. tora (Chakraborty, 2009; Tilwari, 2011).
Conclusion
From the results of present study, it was observed that C. fistula had stimulatory influence on humoral and cell mediated immunity as evident from increased antibody titer against Salmonella Typhimurium ‘O’ antigen and significant enhancement in skin thickness in DNCB sensitized albino rats. HAEL in comparison to HAEP was more effective in stimulating both immune responses. The humoral immune response obtained in HAEL & HAEP fed rats might be due to the presence of quercetin dihydrate within both extracts while increased immune response within HAEL fed rats might be credited to the presence of Kaempferol (Laxmi et al., 2015; Swarnalatha and Puratchikody, 2014).
The augmented humoral and cell mediated immune responses in the presence of HAEP and HAEL of C. fistula reflect the induced proliferation and activation of both B and T lymphocytes. Further studies are although needed regarding digestibility, hepatic toxicity, molecular mechanism of action HAEL & HAEP.
Acknowledgement
The authors are thankful to Prof. A.P. Singh, former V.C. of the Mathura Veterinary University for imparting permission to use necessary facility required during the work. Authors are highly indebted to Dr Ashok Kumar for his constant support and guidance throughout the work performed.
Conflict of interest
The authors state that they have no conflict of interest to declare.
Authors’ contribution
A. K. Bhatia & Anubhuti Sharma conceptualized the study; work was carried out by Vijay Laxmi with the help of Anjana Goel, while Nitin Wahi is credited for formulating and editing the research paper.
References