Journal of Animal Health and Production
Research Article
Study on Efficacy of Diatomaceous Earth to Ameliorate Toxic Effects of Aflatoxin on Internal Organ Weights in Broiler Chicken
Avinash Warundeo Lakkawar1*, Hogalagere Doddappaih Narayanaswamy2, Mayasandra Lakshmikanth Satyanarayana2
1Department of Pathology, Rajiv Gandhi Institute of Veterinary Education and Research (RIVER), Kurumbapet, Pondicherry- 605009, India; 2Department of Pathology, Veterinary College, Bangalore- 560024, Karnataka, India.
Abstract | Diatomaceous earth (DAE) is an abundantly available clay, rich in a morphous silica that have light weight, highly porous and super-absorbent nature. The ability of DAE in reducing the adverse effects of aflatoxin (AF) in broiler diet was evaluated. A total of 240 healthy day old broiler chicks were divided into 6 groups (n= 40 per group), comprising of control and treatment groups. Group I and II was served as negative and positive control respectively. The groups III and IV were given 1 and 0.5 ppm of AF Kg-1 of feed respectively, while groups V and VI were both supplemented DAE @ 2000 mg Kg-1 of feed along with 1 and 0.5 ppm of AF Kg-1 of feed, respectively. Feeding of AF resulted in significant increase (p< 0.05) in the relative weight of liver, kidneys and heart in comparison to the control groups. However, the relative weight of thymus, spleen and bursa was found to be decreased (p< 0.05) in the aflatoxin fed birds. The addition of DAE to the aflatoxin mixed feed significantly improved the relative organ weight and decreased the severity of lesions in the visceral and lymphoid organs. The study concluded that DAE can be effectively used to decrease the toxic effects of AF in broiler chicken.
Keywords | Aflatoxin, Diatomaceous earth, Amelioration, Relative organ weight, Broiler chicken
Editor | Asghar Ali Kamboh, Sindh Agriculture University, Tandojam, Pakistan.
Received | September 08, 2017; Accepted | September 27, 2017; Published | September 30, 2017
*Correspondence | Avinash Warundeo Lakkawar, Department of Pathology, Rajiv Gandhi Institute of Veterinary Education and Research (RIVER), Kurumbapet, Pondicherry- 605009, India; Email: dralakkawar@yahoo.com
Citation | Lakkawar AW, Narayanaswamy HD, Satyanarayana ML (2017). Study on efficacy of diatomaceous earth to ameliorate toxic effects of aflatoxin on internal organ weights in broiler chicken. J. Anim. Health Prod. 5(3): 120-126.
DOI | http://dx.doi.org/10.17582/journal.jahp/2017/5.3.120.126
ISSN (Online) | 2308-2801; ISSN (Print) | 2309-3331
Copyright © 2017 Lakkawar et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Organ weight is one of the most sensitive parameter to study the toxic effect of an experimental compound, as significant differences in organ weight between treatment and untreated (control) groups may occur in the absence of any morphological changes. The comparison of the organ weights of treated with untreated groups is often complicated by differences in body weights between groups. Therefore, other parameters that are commonly used for analysis of organ weight are the ratio of the organ weight to body weight (to account for differences in body weight). These ratios are generally described as relative organ weights (Bailey et al., 2004). All the mycotoxins including aflatoxins (AF) are known to cause adverse alterations in the relative organ weight of visceral and lymphoid organs in animals and birds.
Aflatoxins are secondary metabolites and a class of mycotoxins produced predominantly by Aspergillus flavus and A.parasiticus. These toxins are present worldwide in feeds and cause severe economic lossesto the poultry and livestock industries (Miller, 1995). Studies showed the adverse effects of aflatoxin in broiler chickens including decrease in body weight gain, efficiency of feed utilization, alteration in serum biochemical parameters, relative weights of visceral and lymphoid organs, immune responses and pathological changes in various organs (Ortatatli et al., 2005; Shi et al., 2006; Denli et al., 2009; Khan et al., 2010; Yalagod, 2014; Kumar et al., 2014; Lakkawar et al., 2015).
Producers, researchers and governments aim to develop effective prevention strategies and decontamination technologies to minimise the toxic effects of AF (Rosa et al., 2001). Approaches used have included the physical, chemical and biological treatment of contaminated feed and feed stuffs. A successful detoxification process must be economical and must be capable of eliminating all traces of toxin without leaving harmful residues and also should not impair the nutritional quality of the feed (Parlat et at., 1999).
In the last two decade several studies have been performed using adsorbent materials for detoxifying AF in contaminated feed and feed stuffs (Parlat et al., 1999). Lately, several approaches such as decontamination or remediation of feed and feedstuffs have been proposed (Ledoux et al., 1999). A variety of adsorbents such as zeolite (Miazzo et al., 2000), bentonite (Rosa et al., 2001), hydrated sodium calcium aluminosilicate (Abo-Norag et al., 1995), Saccharomyces cerevisiae (Celik et al., 2001) and activated charcoal (Jindal et al., 1994) have been successfully used in detoxifying AF in contaminated feeds.
Diatomite or diatomaceous earth (DAE) is a kind of clay that consists of 90% silicon dioxide. It is fine-grained, biogenic siliceous sediment, and is abundantly available at low cost (Kamigasa and Kato, 2000). DAE is an inert dust formed by milling of fossilized remains of phytoplankton (diatoms), composed of silicon dioxide, commonly used for the control of insects infesting stored products (Alves, 2006).
Earlier study indicated that DAE is not effective in reducing the detrimental effects of aflatoxin in broiler diets (Denli and Okan, 2006). However, Modirsanei et al. (2008) reported that DAE significantly increased feed intake, body weight +gain, and improved feed conversion ratio as well as productive efficiency index, increased serum albumin, and the activity of serum lactate dehydrogenase in the birds fed AF contaminated feed. Considering the inconsistency of literature regarding the beneficial effects of DAE, the present study was designed to evaluate the efficacy of DAE in ameliorating aflatoxin induced alterations in relative weight of visceral and lymphoid organs in broiler chicken.
MATERIALS AND METHODS
Two hundred and forty unsexed day-old healthy broiler chicks were procured from a reputed commercial hatchery and reared in battery cage system in experimental sheds with average temperature ranging from 27 to 31oC and relative humidity of 59% to 62% with 16:8±I h Light :Dark cycle of intensity of 10 to 20 lux. All chicks were vaccinated on days 7 and 11 of age with the Newcastle disease virus (Lasota strain) and Infectious bursal disease (intermediate strain) respectively.
Optimum conditions of brooding and management was provided to the birds throughout the period of experiment. Toxin free and DAE free starter and finisher broiler feed was procured from Department of Poultry Science, Veterinary College, Bangalore, India. The both starter and finisher feed was formulated as recommended by the National Research Council (NRC, 1994). The DAE was obtained from AGRIPOWER®, Australia. The AF was produced from A. parasiticus MTCC-2796 (Microbial Type Culture Collection and Gene Bank culture) procured from IMTECH, Chandigarh, India via fermentation of rice by the method of Shotwell et al. (1966) with minor modifications. Required quantities of cultured aflatoxin material were added to make the final concentration of aflatoxin in feed to be 0.5 ppm and 1ppm.
The birds were randomly divided into 6 groups, each comprising of 40 chicks. The different experimental group are as per the Table 1.
Table 1: Experimental design for various treatment groups
| Groups | Treatment | No. of birds |
| I |
CONTROL (Toxin free & DAE* free feed) |
40 |
| II |
AGRIPOWER DAE* @ 2000 mg/Kg of feed |
40 |
| III | AFLATOXIN ( 1 ppm)) per Kg feed | 40 |
| IV | AFLATOXIN ( 0.5 ppm)) per Kg feed | 40 |
| V |
AFLATOXIN (1 ppm)) per Kg feed + DAE* @ 2000 mg/Kg of feed |
40 |
| VI |
AFLATOXIN (0.5 ppm)) per Kg feed+ DAE* 2000 mg/Kg of feed |
40 |
* DAE: Diatomaceous earth
The experimental birds were subjected for routine check-up on daily basis for the health and husbandry conditions. Throughout the experimental period, all the sanitary and hygienic precautions were strictly followed. Permission from the Institute Animal Ethical Committee (IAEC) was obtained before the conduct of experiment. A complete record of the clinical manifestations and daily mortality (if any) was also maintained. A total of six birds randomly selected from each group were weighed, euthanized by cervical dislocation and subjected to detailed post-mortem examination on day 7, 14, 21, 28 and 35th day of experiment. The collected organs namely, liver, kidneys, heart, spleen, thymus and bursa of Fabricius were weighed individually using an electronic weighing balance. The relative weight was calculated by the following formula:
Relative weight = organ weight (g) / body weight (g)
The experimental data collected was analysed using the General Linear models (GLM) procedure using Statistical Package for the Social Sciences software 16 (SPSS) of 2010 version. Statements of statistical significance were based on P < 0.05.
RESULTS and DISCUSSION
Mycotoxins, particularly aflatoxin, are detrimental to the poultry industry because of its frequent occurrence in feed stuffs which can adversely affect the health of birds leading to severe economic loss (Kubena et al., 1993). Losses associated with consumption of AF-contaminated foodstuff in the poultry include growth retardation, decreased feed intake, biochemical alterations, patho-morphological changes in various organs, increased mortality, decreased egg production, and carcass condemnation (Khan et al., 2010; Lakkawar et al., 2015; 2017). Aflatoxins at varying dose levels are also known to cause alterations in relative organ weights. A significant alteration in the relative weight of various visceral and lymphoid organs is one of the important toxic effects of aflatoxicosis in chicken (Lakkawar et al., 2015).
Liver is considered the target organ for AF because it is the organ where most aflatoxins are bioactivated to the reactive 8,9-epoxide form, which is known to bind with DNA and proteins, damaging the hepatocytes and increasing liver weight (Pasha et al. 2007). The data furnished in Figure 1 indicatesignificant (p≤ 0.05) increase in the relative weight of liver in aflatoxin treated birds, which corroborated with the earlier reports (Ortatatli et al., 2005; Shi et al., 2006; Denli et al., 2009; Khan et al., 2010; Yalagod, 2014; Lakkawar et al., 2015), which could be attributed to the aflatoxin induced impaired fat metabolism leading to increased accumulation of lipids in the hepatocytes. The hepatic lipidosis is primarily mediated through inhibition of phospholipids synthesis and cholesterol. This in-turn affects the transportation of lipid from the liver (Hsieh, 1979). In addition, vascular, degenerative and inflammatory changes incited by AF in visceral organs might be responsible for increased weights and this observation drew support from the histopathological lesions of severe fatty change, lipidosis and inflammatory reaction reported in an earlier study (Lakkawar et al., 2015).
The graphical representation of data in Figure 2 showed significant (p≤ 0.05) increase in relative weight of kidneys in aflatoxin fed birds. The increased weight of kidneys in the present study corresponded with earlier findings, which could be attributed to vascular reaction and degenerative changes in renal parenchyma following aflatoxicosis (Valchev et al., 2013; Yalagod, 2014; Lakkawar et al., 2015).
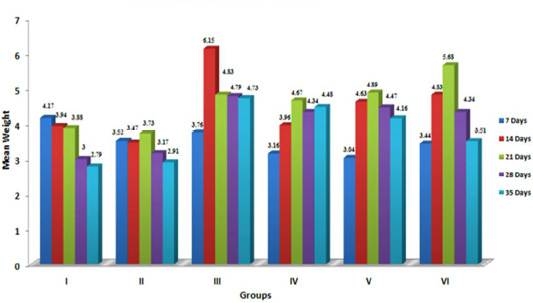
Figure 1: Weekly relative Liver weigh (g) of broiler chicks fed with aflatoxin (AF), Diatomaceous earth (DAE) and their combination
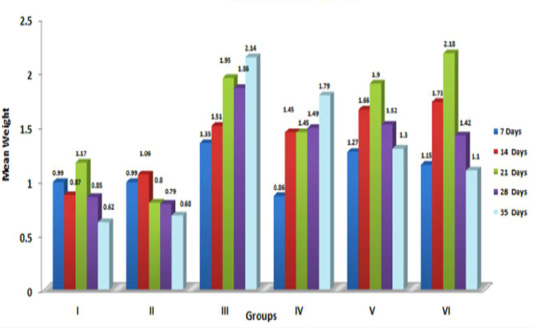
Figure 2: Weekly relative Kidney weight (g) of broiler chicks fed with aflatoxin (AF), Diatomaceous earth (DAE) and their combination
In the present study, the relative weights of thymus, spleen and bursa of Fabricius significantly (p≤ 0.05) decreased in aflatoxin fed birds (Figure 3, 4, 5) and corresponded with earlier reports (Ortatatli and Oguz, 2001; Kumar et al., 2014; Yalagod, 2014; Lakkawar et al., 2015). Decrease in relative weight of lymphoid organs could be attributed to the toxic effect of AF on lymphoid component causing lymphocytolysis leading to decrease in size of lymphoid organs (Peng et al., 2015).
Contrary to the findings of the present study, significant increase in the relative weight of spleen in broilers exposed to aflatoxin-contaminated diets have also been reported by Bailey et al. (2006) and Shi et al. (2006). The variations in relative weights of spleen could be due to various factors like dosage, concentration and duration of exposure of AF, strains of broilers and other nutritional as well as environmental factors.
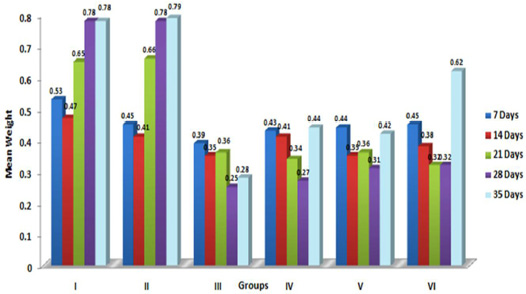
Figure 3: Weekly relative Thymus weight (g) of broiler chicks fed with aflatoxin (AF), Diatonaceous earth (DAE) and their combination
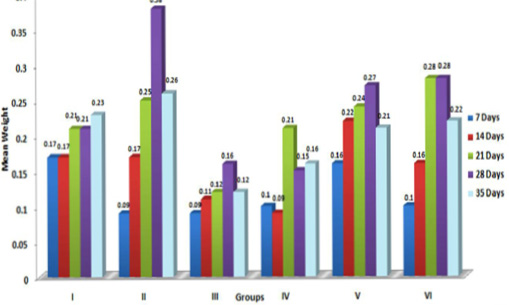
Figure 4: Weekly relative Spleen weight (g) of broiler chicks fed with aflatoxin (AF), Diatomaceous earth (DAE) and their combination
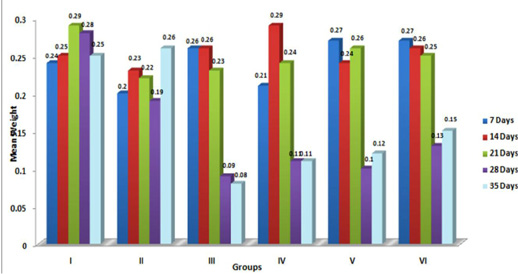
Figure 5: Weekly relative Bursa weight (g) of broiler chicks fed with aflatoxin (AF), Diatomaceous earth (DAE) and their combination
In the present study, significant (p≤ 0.05) increase in the relative weight of heart (Figure 6) is in agreement with the earlier findings of enlargement of heart following aflatoxicosis in broiler birds (Abousadi et al., 2007; Sharghi and Manafi, 2011). A study conducted on broiler chickens following feeding of 0.5 and 0.8 ppm AFB1 for a period of 42 days also revealed significant increase in the relative weight of heart in toxin fed birds (Valchev et al., 2013).
Contrary to the findings in the present study, Modirsanei et al. (2008) reported decreased relative weight of the heart following feeding of aflatoxin @ 1 ppm in diet for a period of 42 days and no reasonable explanation was provided for the decreased weight of heart. However, Kumar et al. (2014) reported no significant change in the relative weight of heart following feeding of 1 ppm of aflatoxin for a period of 35 days. In the present study, increase in the relative weight of heart could possibly be attributed to the vascular and inflammatory reaction observed histologically in aflatoxin fed birds (Lakkawar et al., 2015).
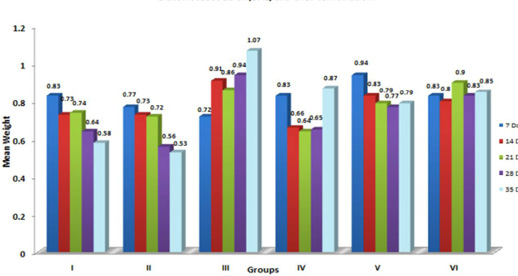
Figure 6: Weekly relative Heart weight (g) of broiler chicks fed with aflatoxin (AF), Diatomaceous earth (DAE) and their combination
Studies have been carried out to reduce the toxicity of AF aimed towards binding as well as adsorption of aflatoxin in the gastrointestinal tract (Schell et al., 1993). The major advantage of these adsorbents includes cost effectiveness, safety and ease of administration through feed. A variety of zeolites, phyllosilicates and synthetic aluminosilicates are also capable of removing AFs from aqueous solutions however, major difference can be observed in the strength of aflatoxin binding by the above adsorbents (Abdel-Wahhab et al., 1999).
In the present study, diatomaceous earth was used for amelioration of aflatoxin in poultry diet. Diatomaceous earth refers to a naturally-formed sedimentary mineral obtained from the remains of what were once oceanic unicellular shells and algae known as diatoms. These organisms, much like a mollusc emits lime-carbonate, had the ability to emit silica. These clay-like, chalky remains are referred as diatomite (Eralsan et al., 2006). Diatomaceous earth, a clay mineral of the smectite group is formed of highly colloidal material composed of mainly montmorillonite and is produced by in-situ diversification of volcanic ash. It has the unique characteristic of swelling to several times its original volume when placed in water and of forming thixotropic gels with water even though the amount of clay is relatively less. DAE may possess higher adsorptive capacity due to its structural composition, which can be responsible for the binding to mycotoxins in the feed materials (Parker, 1988).
The birds in co-treatment group (AF+ DAE) showed significant (p≤ 0.05) reduction in relative weight of liver, kidneys and heart, and significant (p≤ 0.05) increase in relative weight of thymus, spleen and bursa of Fabricius in comparison to aflatoxin alone fed birds (Figure 1,2,3,4,5,6). Similar findings have also been reported by earlier workers (Shi et al., 2006; Bailey et al., 2006; Denli et al., 2009, Eckhardt et al., 2014), which could be related to possible role of DAE in ameliorating adverse effects of aflatoxin, as indicated earlier by its absorbent property. However, no change in relative weight of liver, kidney, spleen and bursa of Fabricius in response to incorporation of DAE in AF mixed feed of broilers has also been reported earlier (Modirsanei et al., 2008).
Contrary to our findings, no protective effect of DAE on adverse effects of AF on various organs of birds has also been reported (Shi et al., 2006; Denli et al., 2009). Differences among these studies could be explained by different levels of adsorbents or the AF exposure dose tested. Based on the available literature, the chemical complexity of mycotoxins means that a compound’s effectiveness in sequestering one mycotoxin does not mean an equal ability to sequester other mycotoxins. Each mycotoxin has different functional groups; thus, the binding capacity of an adsorbent depends on its chemical and physical properties and its relation with the physical structure of the target mycotoxins. Thus, the physicochemical differences among the adsorbents used in the studies mentioned above could explain the higher or lower efficacy among them. However, the ability of the toxin binder to bind mycotoxins depends on other factors such as pH, molecular arrangement and its geographic region of origin (Vieira, 2003). Natour and Yousef, (1998) reported significantly higher in-vitro adsorption ability of DAE to aflatoxin, which is directly proportional to the number of diatom valves. In- vitro study showed that DAE has high (94.71 %) ability to adsorb AF from the feed at pH 6.5 (Soleimani et al., 2011).
In summary, the incorporation of DAE in the diet during the period of exposure to AF in the present study could considerably reduce the toxic effects of aflatoxin. This study highlights the protective effects of DAE, which might be due to its higher specific chemisorption capability of aflatoxin in gastrointestinal tract similar to that of aluminosilicate compounds, which reduces AF bioavailability by formation of aflatoxin-DAE complex followed by excretion through droppings/faeces (Phillips et al., 1988; Abdel-Wahhab et al., 1999).
In respect of abundant availability, source and cost of DAE as well as ability of this absorbent in reducing the moisture content in the poultry litter (Kiaei et al., 2002), it seems that using DAE at the level of 2000mg kg-1 of diet could be effective and economic way of ameliorating the adverse effect of aflatoxin in poultry feed.
Perusal through the documented literature suggest that clay, zeolite minerals and DAE are structurally and functionally diverse; they vary considerably from source to source and may not have equal affinities and capacities for aflatoxin and other mycotoxins, thus they should be rigorously tested one by one and thoroughly characterised in vivo, paying particular attention to their effectiveness and safety for sensitive animal models and their potential for harmful interactions. Similarly, generalisations should be avoided for all potential mycotoxins detoxifying agents, as adsorbing compounds can differ in efficacy even within the same category. Considering the results of earlier work done on effect of different levels of DAE on aflatoxin, further studies seems to be necessary to determine whether lower levels of DAE in broilers diet will be effective in controlling or preventing the occurrence of aflatoxicosis in chicken.
CONCLUSION
In the present study, aflatoxin in the broiler diet at level 0.5 ppm kg-1 and 1 ppm kg-1 of feed affected the relative organ weights indicating their adverse effect on general health of the experimental birds .Incorporation of DAE at level of 2000 mg kg-1 of toxin free broiler feed showed no adverse effects on relative organ weights as compared to healthy control. The addition of Agripower DAE to aflatoxin containing feed revealed significant improvement in relative organ weight. The protective effect of 2000 mg of Agripower DAE Kg-1 feed could be useful to counter aflatoxicos is problem in field conditions that is probably due to its higher binding ability.
ACKNOWLEDGEMENTS
Authors are the thankful to AGRIPOWER, Australia for providing DAE and financial support from ICAR-NAE project and the Dean, Veterinary College, Bangalore, India for providing the necessary facilities to carry out the research work.
Conflict OF INTERESTS
The authors have no conflicts of interest to declare.
AUTHORS CONTRIBUTION
Authors contributed equally and have read and approved the final manuscript.
REFERENCES





