Journal of Animal Health and Production
Research Article
Prevalence of Trypanosoma brucei brucei and Potential In Vitro Trypanocidal Activity of Aqueous Extracts of Some Medicinal Plants in the Pastoral Area of Gaongho in Burkina Faso
Zongo André1,4, Kaboré Adama1*, Bengaly Zakaria2, Vitouley Sèna Hervé2, Traoré Aristide3, Tamboura Hamidou Hamadou1, Belem Adrien Marie Gaston4
1Département Productions Animales / Institut de l’Environnement et de Recherches Agricoles (INERA), 04 BP 8645 Ouagadougou 04, Burkina Faso; 2Centre International de Recherche-Développement sur l’Elevage en zone Subhumide (CIRDES), 01 BP 454 Bobo-Dioulasso 01, Burkina Faso; 3Institut de Recherche en Sciences de la Sante (IRSS/CNRST) 01 BP 7192 Ouagadougou 01 Burkina Faso; 4Institut du Développement Rural (IDR)/Université Nazi Boni, 01 BP 3770 Ouagadougou 01, Burkina Faso, West Africa.
Abstract | In the pastoral zone of Gaongho in Burkina Faso, the main disease of ruminants is the African Animal Trypanosomiasis (AAT) which is very often controlled by herders with the aqueous extracts of five plants whose present study proposes to evaluate the potential trypanocidal activities. To this end, a parasitological field survey in ruminants was carried out followed by an in vitro trypanocidal test of five plant extracts using three concentrations (25, 50 and 100 mg / ml) in triplicate in comparison with negative (PBS) and positive (Veriben) controls in the laboratory. The field survey revealed an overall prevalence rate of 4.14% for trypanosomes, including 4.54% for cattle and sheep and 3.33% for goats, with no significant difference (P ˃ 0. 05) was recorded between the three ruminant species. Among the latter, the sex effect showed a significant difference in goats where males were more infested (P = 0.0015) than females. On the other hand, the age of the animals presented no significant difference (P = 0.8218) between the three species. All ruminants tested were more infested with Trypanosoma brucei brucei (66.67%) than T. congolense (33.33%). The in vitro test was conducted with aqueous extracts of five plants (Balanites aegyptiaca, Capparis sepiaria, Guiera senegalensis, Mitragyna inermis, and Vitellaria paradoxa) on Trypanosoma brucei brucei. Concentrations of 50 and 100 mg / ml of G. senegalensis leaf extracts and V. paradoxa bark caused high mortality of T. brucei brucei and were comparable to Veriben, the standard reference product. The results obtained with the extracts of G. senegalensis and V. paradoxa showed that these plantsmay be the potential source of trypanocidal drugs.
Keywords | In vitro, Prevalence; Trypanocidal activity, Medicinal plants, Gaongho pastoral area, Burkina Faso
Editor | Asghar Ali Kamboh, Sindh Agriculture University, Tandojam, Pakistan.
Received | September 08, 2017; Accepted | September 27, 2017; Published | September 30, 2017
*Correspondence | Kaboré Adama, Département Productions Animales / Institut de l’Environnement et de Recherches Agricoles (INERA), 04 BP 8645 Ouagadougou 04, Burkina Faso, West Africa; Email: ade1_bf@yahoo.fr
Citation | Andre Z, Adama K, Zakaria B, Herve VS, Aristide T, Hamadou TH, Gaston BAM (2017). Prevalence of trypanosoma brucei brucei and potential in vitro trypanocidal activity of aqueous extracts of some medicinal plants in the pastoral area of gaongho in burkina faso. J. Anim. Health Prod. 5(3): 107-114.
DOI | http://dx.doi.org/10.17582/journal.jahp/2017/5.3.107.114
ISSN (Online) | 2308-2801; ISSN (Print) | 2309-3331
Copyright © 2017 Andre et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Animal African Trypanosomiasis (AAT) are one of the major constraints to the development of livestock and consequently to the improvement of rural farmers’ income in Burkina Faso, a country of West Africa (Sow et al., 2010). In order to cope with this pathology, numerous anti-vector campaigns have recently been implemented under the Pan-African Tsetse and Trypanosomosis Eradication Campaign (PATTEC) in Burkina Faso, Mali and Ghana (Sow et al., 2012; Adam et al., 2013; Dicko et al., 2015).The eradication of AAT vectors which is the initial objective of the PATTEC could not be achieved in addition to being expensive and laborious. In fact, the treatment of cattle with trypanosomosis continues to focus mainly on the use of the two main compounds used for decades, ie diminazene aceturate and isometamidium chloride (Mamoudou et al., 2006; Mamoudou et al., 2008; Delespaux et al., 2008a; Vitouley et al., 2013; Tchamdja et al., 2016). Unfortunately, many cases of resistance to these compounds have been detected in more than 18 African countries (Delespaux et al., 2008a; Vitouley et al., 2013; Tchamdja et al., 2017). In addition, the development of vaccines in the near future being uncertain (Magez and Radwanska, 2009; Magez et al., 2010), alternative research approaches including study of trypanocidal medicinal plants commonly used by rural farmers are increasingly explored (Vitouley et al., 2017).
In Burkina Faso, particularly in the pastoral area of Gaongho, livestock farmers/herders often use natural plant resources called medicinal plants grown in their areas to treat different animal diseases (Tamboura et al., 1998; CAPES, 2006; Okoli et al., 2010).This use of traditional herders’ medicine is popular due to the fact that the chemical drugs recommended for the control of parasites are expensive (Kaboré et al., 2007).
According to Zongo et al. (2017), herders in Gaongho pastoral area use many local herbal medicinal recipes to fight against AAT. The plants commonly used are Balanites aegyptiaca, Capparis sepiaria, Guiera senegalensis, Mitragyna inermis, and Vitellaria paradoxa. In order to scientifically verify the herders’ claims, this study aims to (i) conduct a cross-sectional survey to identify the main dominant trypanosome species in ruminants in the Gaongho pastoral zone and (ii) to evaluate in vitro potential trypanocidal effects of the aqueous extracts of these five medicinal plants mentioned above.
MATERIALS AND METHODS
In the study, two experiments were carried out, namely the evaluation of the trypanosome prevalence in Gaongho pastoral area and the in vitro test of the potential trypanocidal effects of the five plant extracts used by herders.
Experiment 1: Trypanosome Prevalence
Study area: Gaongho pastoral area covers an area of 6762 hectares and is located in the province of Bazèga with the coordinates 11°56’ and 12°04’ North latitude and 01°03’ and 01°09’ West longitude (MRA, 2000). The climate of the area is north-Sudanian domain where a rainy season is from May to October and a dry season from November to April. Temperatures are very variable with an average of 30°C. The rainy season is characterized by abundant rainfall but poorly distributed over time, whereas in the dry season, there is a strong sunshine with high heat (MATD, 2007). This situation leads very quickly to the drying up of the few natural water reservoirs and negatively impacts the agricultural production activities. The vegetation of the area is a grassy and wooded savanna. The trees are medium in size and consist of Vitellaria paradoxa, Sclerocarya birrea, Lannea acida, Lannea microcarpa, Piliostigma thonningii, Piliostigma reticulatum, Balanites aegyptiaca, Ziziphus mucronata, Ximenia americana, Acacia seyal, Guiera senegalensis, etc. The herbaceous stratum is comprised of Aristida adscensionis, Andropogon gayanus, Andropogon acinodis, Pennisetum violaceum, Pennisetum pedicellatum. The resident population is composed of Fulani (mostly) and Mossi, who rely mainly on livestock and agriculture.
Methodology applied: The field survey was carried out from October 2014 to September 2015 by taking blood samples from ruminants (cattle, sheep and goats) naturally exposed to trypanosome infestation in Gaongho pastoral area. These samples were taken at random from the animals, followed by the collection of individual information on the mode of rearing, age (young≤1 year and adult >1 year in small ruminants, and young ≤3 years and adult >3 years in cattle), sex and race. At the level of each animal, the blood was taken from the jugular vein in tubes containing EDTA (Desquesnes and Dia, 2004).Blood smears were taken on pastoral area and then sent to the laboratory where they were stained at May-Grünwald Giemsa with a rapid staining kit (Kit RAL 555® - RAAL DIAGNOSTIC, France). The slides were then observed under the microscope at magnification x 40 to determine the presence of trypanosomes and to identify their species (Trypanosoma congolense, Trypanosoma vivax and Trypanosoma brucei).Concomitantly, the trypanocidal plants according to the herders were harvested on site in April 2015 for in vitro test in laboratory.
Experiment 2: In Vitro Test
Preparation of aqueous extracts: Seven extracts (macerated and decoction) of plants from the five plants (Vitellaria paradoxa, Mitragyna inermis, Balanites aegyptiaca, Capparis sepiaria and Guiera senegalensis) were prepared in accordance with the herders’ procedure (Zongo et al., 2017). These preparations have been adapted to the current techniques of maceration and decoction at the “Institut de Recherche en Sciences de la Santé (IRSS)” to conduct the trypanocidal test in vitroin“Centre International de Recherche-Développement sur l’Elevage en zone Subhumide (CIRDES)”.
For the maceration of the extracts, 200 g of the sample of each plant material was placed in a 2000 mL Erlenmeyer flask containing a volume of 1500 mL of distilled water. The mixture was homogenized with a glass rod and then subjected to magnetic stirring for 24 h at room temperature (25-30 ° C). The extract was then filtered by pressing with a nylon cloth. The marc obtained is rinsed with 500 mL of hot water. Obtained filtrates were centrifuged at 2000 rpm for 10 minutes. The supernatant after centrifugation (macerate) is collected and concentrated at 45°C in a ventilated oven. The concentrated extract obtained is transferred to freezing jars for freeze-drying.
For the decoction of the extracts, 200 g of the sample of the plant material was placed in a 2000 mL beaker equipped with a lid in which a volume of 2000 mL of distilled water was added. The mixture was homogenized with a glass rod and then boiled on a hot plate for 1 h (mechanical stirring with a glass rod was made from time to time). After, the extract was warmed and then filtered by pressing on a fine nylon cloth. Obtained filtrate was then centrifuged at 2000 rpm for 10 minutes, the supernatant was collected and concentrated at 45°C in a ventilated oven. The concentrated extract obtained was transferred to freezer jars for freeze-drying.
Infection of mice: Trypanosoma brucei brucei strain (reference Farakoba 80 / CRTA / 01) was got from the “Centre International de Recherche-Développement sur l’Elevage en zone Subhumide (CIRDES)” and stored in liquid nitrogen. These strains were revitalized for two minutes in a 25°C water bath. After checking the viability of the stabilates, 15 NMRI mice previously irradiated were infected. Following the method of Herbert and Lumsden (1979), the mice were anesthetized with ether and the blood was collected by cardiac puncture in a heparinized tube when the parasitemia reached 109 trypanosomes per ml of blood for carrying out the in vitro trypanocidal test.
In vitro trypanocidal test: The test was carried out in triplicate in a microplate of 96 wells according to the procedure of Herbert and Lumsden (1976). To this end, 50 μL of blood containing 106 trypanosomes (Trypanosoma brucei brucei) per ml were incubated with 50 μL of each plant extract containing three increasing concentrations (25, 50 and 100 mg / ml). At the same time, two controls were made, one of which was positive control with a conventional product containing diminazene aceturate (Veriben) used to validate the test and a negative control with a buffered solution (Phosphate Buffered Saline: PBS). The level of parasitaemia was followed at 05 min, 15 min and 30 min by observation of 0.5 μl of blood under the x40 objective microscope. The decrease or cessation of the motility of the parasites in the blood subjected to the tested extracts and to the controls was used to measure the trypanocidal activity.
Statistical Analysis
Data on field survey were recorded by species, sex and age before being subjected to descriptive analysis assessing prevalence and the Chi2 test to discriminate the measured parameters. In vitro data were expressed as mean ± SD and subjected to ANOVA (one-way) in order to compare the means obtained by the Tukey-Kramer test at 5%. Before, the in vitro data were transformed (log (x + 1)) to normalize their distribution.
RESULTS
Trypanosome Prevalence
Table 1 shows an overall prevalence rate of 4.14% AAT in ruminants in the pastoral area, whereas sheep and cattle were the most infected (4.54%) compared to goats (3.33%). These prevalence rates did not reveal any significant difference (P = 0.8621) between the three ruminant species studied at which the sex effect had a significant difference (P = 0.0350), particularly in goats where males were significantly more infected (P = 0.0015) than females.
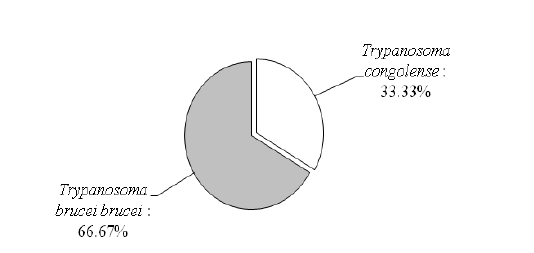
At the age of the animals, adults were more infected (4.30%) than the young (3.92%) with no significant difference (P = 0.8218) between all ruminants and within each ruminant specie. All AAT-induced infections to ruminants were mono-specific and caused by Trypanosoma brucei brucei and T. congolense, with a clear dominance of T. b. brucei infections (Figure 1).
Plants Extracts Extraction Yield
The yields of the seven extracts of the five trypanocidal plants commonly used in the treatment of Trypanosome in ruminants by herders in Gaongho pastoral area are shown in Table 2. The mean values obtained ranged from 9.14% with the Balanites aegyptiaca bark to 35.62% with the Mitragyna inermis leaves. The leaves of M. inermis in decoction and B. aegyptiaca in maceration obtained the high yields which are 35.62% and 32.07%, respectively. The lowest yield was obtained with the decoction of B. aegyptiaca bark (9.14%).
In Vitro Trypanocidal Activity of Plant Extracts
Table 3 presents the results of in vitro trypanocidal activity on T. b. brucei of the seven aqueous extracts of five plants.
Table 1: Trypanosome prevalence rate in ruminants in relation to species, sex and age in the Gaongho pastoral area
|
Parameters |
Animals | Chi2 | dl | P(Chi2) | |||
|
Total examined |
Negative |
Positive (%) |
|||||
|
Species |
Cattle | 110 | 105 | 5 (4.54) | 0.297 | 2 | 0.8621 |
| Goat | 120 | 116 | 4 (3.33) | ||||
| Sheep | 132 | 126 |
6 (4.54) |
||||
| Total | 362 | 347 | 15 (4.14) | ||||
| Sex | Females | 293 | 284 | 9 (3.07) | 4.447 | 1 | 0.0350 |
| Males | 69 | 63 | 6 (8.69) | ||||
| Total | 362 | 347 | 15 (4.14) | ||||
| Age | Youngs | 155 | 149 | 6 (3.92) | 0.051 | 1 | 0.8218 |
| Adults | 207 | 198 | 9 (4.30) | ||||
| Total | 362 | 347 | 15 (4.14) | ||||
Table 2: Dry extract yields of the samples of the medicinal plants
| Names of plants | Used parts | Methods of preparation | Yields (%) |
|
Balanites aegyptiaca
|
Bark | Decoction | 9.14 |
| Leaves | Maceration |
32.07 |
|
| Capparis sepiaria | Roots | Decoction | 19.40 |
| Guiera senegalensis | Leaves | Decoction | 19.46 |
| Mitragyna inermis | Leaves | Decoction | 35.62 |
|
Vitellaria paradoxa
|
Bark | Maceration | 9.87 |
| Leaves | Maceration |
26.04 |
During the test, the negative reference control (PBS) and the extract of Capparis sepiaria showed no inhibition or mortality of the parasite. On the other hand, the percentages of T. b. brucei mortality of the active extracts increased with increasing concentrations.
Indeed, the positive control (diminazene aceturate), Guiera senegalensis leaf extracts and Vitellaria paradoxa bark at concentrations of 50 mg/ml and 100 mg/ml and Balanites aegyptiaca bark at 100 mg/ml showed significant efficacy (P ˂ 0.05) on T. b. brucei after 5 min compared to the other extracts and the negative control (PBS).
At 15 min incubation, concentrations of 50 mg / ml and 100 mg / ml induced percentages of T.b. brucei mortality of 96.6% and 100% respectively for G. senegalensis leaves, 100% for V. paradoxa bark and the positive control (Veriben) and 3.3% and 50% respectively for B. aegyptiaca bark. At 100 mg/ml, the aqueous extracts of M. inermis and B. aegyptiaca leaves had a significantly high effect (P ˂ 0.05) on T. b. brucei than the control PBS.
At 25 mg/ml, extracts from G. senegalensis leaves and B. aegyptiaca bark caused no effect on T. b. brucei throughout the duration of the test. While this same concentration of 25 mg/ml caused T. b. brucei mortalities of 56.6% and 100% at 15 min and 76.6% and 100% at 30 min incubation with the extracts of V. paradoxa bark and the positive control (Veriben) respectively.
DISCUSSION
This study was carried out in Burkina Faso in the pastoral area of Gaongho where the population is mainly composed of livestock keepers who mainly depend on their pastoral activities based on ruminant rearing. The livestock of these animals are cattle, sheep and goats that help herders to derive most of their subsistence to satisfy socio-economic needs of their families. The actions of herders in the study area are hampered by the persistence of AAT transmitted by tsetse flies and other mechanical actions, thus impacting the social welfare of herders (ADF, 2004).
During the study in the pastoral area of Gaongho, serological tests carried out on ruminants revealed an overall prevalence rate of 4.14% AAT, of which 4.545% in cattle and sheep and 3.33% in the goats was observed.
These rates in cattle and sheep are large but lower than in cattle in the north (10.2%) by Tchamdja et al. (2017) and in the Mô Plain (37.64%) by Talaki et al. (2014) in Togo, in sheep (13.1%) by Farougou et al. (2011) in southern Guinean zone of Benin and in goats (8%) by Bastiaensen (2003) in Sokodé in Togo. It is also lower than the 6.86% observed by Degnah et al. (2013) in Ethiopia in the Lalo Kile district and above the 2.5% obtained by Adam et al. (2012) in Ghana in cattle.
In the cattle and ovine, the sex effect observed during the study is contrary to the results of the studies of Tola et al. (2016) in Ethiopia in cattle and Farougou et al. (2011) in sheep where females were more infected than males. On the other hand, our prevalence values recorded by species of small ruminants are higher than those obtained by Sam
Table 3: Mean percentages (mean ± SD) of T. brucei brucei mortality subjected to plant aqueous extracts compared to controls (Veriben and PBS)
| Plant extracts | Concentrations (mg/ml) | Observation time (minutes) | ||
| 5 | 15 | 30 | ||
| MIl | 25 | 0±0 b | 0±0 c | 0±0 d |
| 50 | 0±0 b | 0±0 c | 0±0 d | |
| 100 | 0±0 b | 41.6±4.8 abc | 71.6±28.4 ab | |
| VPb | 25 | 0±0 b | 56.6±4.04 ab | 76.6±25.1 a |
| 50 | 88.3±16.0 a | 100±0 a | 100±0 a | |
| 100 | 100±0 a | 100±0 a | 100±0 a | |
| VPl | 25 | 0±0 b | 0±0 c | 0±0 d |
| 50 | 0±0 b | 0±0 c | 0±0 d | |
| 100 | 0±0 b | 13.3±2.3 bc | 21.6±3.7 cd | |
| BAb | 25 | 0±0 b | 0±0 c | 0±0 d |
| 50 | 3.3±0.57 b | 3.3±0.57 c | 10±1.0 bcd | |
| 100 | 43.3±11.5 a | 50.0±2.0 ab | 68.3±7.6 ab | |
| BAl | 25 | 0±0 b | 0±0 c | 0±0 d |
| 50 | 0±0 b | 3.3±0.57 c | 3.3±0.57 cd | |
| 100 | 3.3±0.57 b | 26.6±3.7 abc | 36.6±3.5 abc | |
| GSl | 25 | 0±0 | 0±0 c | 0±0 d |
| 50 | 96.6±5.7 a | 96.6±5.7 a | 100±0 a | |
| 100 | 70±5.1 a | 100±0 a | 100±0 a | |
| CSr | 25 | 0±0 b | 0±0 c | 0±0 d |
| 50 | 0±0 b | 0±0 c | 0±0 d | |
| 100 | 0±0 b | 0±0 c | 0±0 d | |
| Veriben | 25 | 00±00 b | 100±00 a | 100±00 a |
| 50 | 93.3±11.5 a | 100±00 a |
100±00 a |
|
| 100 | 100±0 a | 100±00 a | 100±00 a | |
| PBS | 25 | 0±0 b | 0±0 c | 0±0 d |
| 50 | 0±0 b | 0±0 c | 0±0 d | |
| 100 | 0±0 b | 0±0 c |
0±0 d |
|
(abcd) Means with different letters on the same column are significantly different (P <0.05).
BAb: Balanites aegyptiaca bark; BAl : Balanites aegyptiaca leaves; CSr:Capparis sepiaria roots; GSl: Guiera senegalensis leaves; MIl: Mitragyna inermis leaves; VPb : Vitellaria paradoxa bark;VPl: Vitellaria paradoxa leaves PBS: Phosphate buffer saline
di et al. (2010) at the Kaduna slaughterhouse in Nigeria (1.54%) where males (0.57%) were less infected than females (2.92%) in goats. As for the age effect of the ruminants surveyed, adults were more infested (4.30%) than the young (3.92%), corroborating the observations made in Ethiopia in cattle by Tola et al. (2016) and in Benin in sheep by Farougou et al. (2011). All infected ruminants showed higher infections with T. b. brucei (66.67%) than Trypanosoma congolense (33.33%) and mono-specific. This predominance of T. brucei is similar to that noted by Adam et al. (2012) in Ghana but diverges from the clear dominance of T. congolense in Ethiopia by Tola et al. (2016) and Degnah et al. (2013) in Ethiopia and T. vivax by Talaki et al. (2014) in Togo in their studies. All of these differences in prevalence among the authors reveal a high variability of AAT infections in ruminants depending on the density of tsetse and other biting insects and the ability of animals to develop high parasitaemia.
In the study, the overall prevalence observed in ruminants appears to be relatively low in the Gaongho pastoral area. This could be explained by the effects of climate change and anthropogenic actions on natural resources in the area in recent years that have contributed to the current transformation of tsetse ecology for their survival (Dao et al. al., 2008; Van den Bossche et al., 2010). This could be added to the diagnostic method used in the study which is less sensitive than the PCR technique using parasite-specific primers which is more sensitive and allows to have higher prevalences (Desquesnes et al., 1999; Marcotty et al., 2008).
In the pastoral area, all animals infected with the AAT are generally treated with chemotherapy with the help of public and private veterinary services and with the use of medicinal plants by the herders themselves or the traditional-practitioners (Zongo et al., 2017).
The yields of the seven extracts commonly used by herders in the pastoral area and tested in the present study range from 9.14% to 35.62%. Bark extracts showed the lowest yields (9.14% - 9.87%) compared to roots (19.40%) and leaves (19.46% - 32.07%). The differences in yield between the seven extracts of five plants are probably due to the fact that at the time of harvesting the materials studied the leaves had lower water content than the bark and roots of the plants. In addition, it may be suggested that there are more polar compounds in the leaves than in the barks and roots of harvested plants. Subsequent further studies of phytochemical screening of our plant extracts would confirm our assertion. The yields of our seven extracts are significantly higher than those obtained by Yaro (2016), which yielded green and bicolored fruits of S. aethiopicum prepared under the same conditions yields of 8.69% and 9.00% respectively. The difference between the two studies is due to the parts used for the preparation of extracts which are leaves, roots and bark in the study in the pastoral area of Gaongho.
The in vitro test carried out in the study showed the advantages of being simple to perform, relatively inexpensive, reproducible and requiring only a small amount of biological material. The results obtained show that aqueous extracts have an appreciable trypanocidal effect on T. b. brucei in the following order: Vitellaria paradoxa bark ˃ Guiera senegalensis leaves ˃ Balanites aegyptiaca bark. All these active extracts on T. b. brucei act in a dose-dependent manner and in relation to time. The results of their trypanocidal activity are similar to that of diminazene aceturate, which contains a single pure active principle commonly used for the conventional treatment of trypanosomiases. On the other hand, aqueous extracts usually contain several secondary metabolites that act synergistically or individually on pathogenic organisms (Cowan, 1999) and especially on trypanosomes (Bala et al., 2010). Compared to these active extracts, those of Mitragyna inermis and B. aegyptiaca leaves showed a weak trypanocidal effect only at the concentration of 100 mg/ml on T. b. brucei. Conversely, extracts from V. paradoxa leaves and Capparis sepiaria roots showed no trypanocidal effect as Cissus quadrangularis on T. b. brucei in Nigeria in the study of Bala et al. (2009). Our results are consistent with Atawodi et al. (2003), Hoet et al. (2004) and Mann et al. (2009) who reported the in vitro trypanocidal activity of some medicinal plants on trypanosomes in their studies. The difference of the in vitro trypanocidal activity on T. b. brucei between our seven aqueous extracts tested could be explained by: (1) the nature of the secondary metabolites contained in each extract and (2) the concentration of the active substance (s) responsible for the observed trypanocidal activity during the test. Hence there is great need to carry out the screening of the phytochemicals contained in the extracts studied, particularly the most active (V. paradoxa barks and G. senegalensis leaves) in order to better understand their mechanisms of actions on T. b. brucei. Indeed, it is known that many natural products exhibit their trypanocidal effect by interfering with the redox balance of the parasites by acting either on the respiratory chain or on the cell defenses against oxidative stress (Adeiza et al., 2010). Maikai (2008) reported that natural products possess structures capable of generating radicals that can lead to peroxidative damage to trypanothione reductase which is very sensitive to alterations in redox balance. Other secondary metabolites act by binding to the parasite’s kinetoplast DNA (cDNA) (Atawodi et al., 2003, Liu et al., 2005).
CONCLUSION
In the study, aqueous extracts of G. senegalensis leaves and V. paradoxa bark showed the highest trypanocidal activities, unlike the other plant extracts on T. brucei brucei. The trypanocidal effects of their extracts were comparable to the positive control of diminazene aceturate, the conventional reference product. Therefore, these active plants could be a source of novel trypanocidal compounds. These results of the in vitro study demonstrate that traditional medicine can be effective as modern medicine and as an alternative solution in the fight against AAT. However, further research is needed to characterize the secondary metabolites contained in the extracts of these plants and to conduct in vivo tests to advise and secure herders in their uses.
ACKNOWLEDGMENTS
The authors are grateful to the project FCN 05-2014 of PPAAO/WAAPP of Burkina Faso for financial support provided and farmers in the Gaongho pastoral area for their participation in the study.
conflIct of IntErESt
There is no conflict of interest among the authors.
AutHorS contrIButIon
All the authors contributed significantly to the paper. Authors Tamboura H. H, Kaboré A., Belem A. M. G. carried the study and the protocols, Zongo A. and Viyouley S. H. collected the data, Kaboré A., Bengaly Z. and Viyouley S. H. helped in statistical analysis and interpretation of results, all authors contributed to the writing and revision of the paper.
REFERENCES