Journal of Animal Health and Production
Research Article
Prevalence and Risk Factor Analysis of Bovine Tuberculosis in Bovine Population in Karachi, Pakistan
Mujeeb-ur-Rehman Memon1*, Abdul Latif Bhutto1, Pershotam Khatri2, Muhammad Ghiasuddin Shah3, Muhammad Ismail Memon1
1Department of Veterinary Medicine, Sindh Agriculture University, Tandojam, Pakistan; 2Department of Animal Reproduction, Sindh Agriculture University, Tandojam, Pakistan; 3Department of Anatomy and Histology, Sindh Agriculture University, Tandojam, Pakistan.
Abstract | Bovine tuberculosis (bTB) is a major infectious disease among cattle, other farm animals, and human population. It results from infection ofMycobacterium bovis.A cross-sectional study was conducted on 1000clinically suspected bovine animals (435 cows and 565 buffaloes) of smallholder dairy farms in five towns i.e., Bin Qasim, Gaddap, New Karachi, Korangi and Super highway of Karachi, Pakistan. The prevalence of bTB was determined through comparative intradermal tuberculin (CIDT) test using mammalian tuberculin.Asurvey was also conducted to collect information regarding bTB associated risk factors both at herd and animal level. Results showed an overall prevalence of 14.4% bTB in small holder dairy farms of Karachi. The prevalence percentage was significantly (p<0.05) lower in New Karachi (5%) as compared to Gaddap (12.5%) followed by Korangi (15.5%), Super Highway (19%) and Bin Qasim (20%). Pearson’s chi-square revealed that herd level prevalence of bTB was significantly associated withherdsize (χ2= 2.66, p= 0.000) and management system of the farm (χ2= 5.16, p= 0.023). However, animal level prevalence was found associated with the age (χ2=81.14, p=0.000), body condition(χ2=18.74, p= 0.001), sex (χ2=1.101,p = 0.005), breed(χ2=3.83, p=0.003), reproductive status (χ2=26.63, p= 0.009) and origin of animal(χ2=8.27, p= 0.008).Based on CIDT results it could be concluded that bTB is present in significant proportions in bovine population in peri-urban areas of Karachi. The study underlines the importance of launching control strategy before the disease reaches its climax and pose great economic and public health hazard.
Keywords | Bovine tuberculosis, Prevalence, Karachi, Risk factors
Editor | Asghar Ali Kamboh, Sindh Agriculture University, Tandojam, Pakistan.
Received | March 13, 2017; Accepted | April 21, 2017; Published | April 23, 2017
*Correspondence | Mujeeb-ur-Rehman Memon, Department of Veterinary Medicine, Faculty of Animal Husbandry and Veterinary Sciences, Sindh Agriculture University, 70060 Tandojam, Pakistan; Email: mujeeb61_sau@hotmail.com
Citation | Memon MR, Bhutto AL, Khatri P, Shah MG, Memon MI (2017). Prevalence and risk factor analysis of bovine tuberculosis in bovine population in Karachi, Pakistan. J. Anim. Health. Prod. 5(2): 44-49.
DOI | http://dx.doi.org/10.17582/journal.jahp/2017/5.2.44.49
ISSN (Online) | 2308-2801; ISSN (Print) | 2309-3331
Copyright © 2017 Memon et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Bovine tuberculosis (bTB) is a chronic and contagious disease of cattle and other domestic and wild animals, including human (Radostits et al., 2007). Mycobacterium bovis (M. bovis) is a causative agent which is a member of the Mycobacterium tuberculosis complex, a group of mycobacterial species that includes M. tuberculosis, M. bovis, M. africanum and M. microti. From those, M. bovis is the most universal pathogen for the disease bovine tuberculosis among Mycobacterium species and affects many vertebrate animals of all age groups, including humans, although, cattle, goats and pigs are found to be most susceptible, while sheep and horses are showing a high natural resistance and the disease is characterized by progressive development of tubercles in any tissue/organ of the body (Romha et al. 2013; Ameni, 2004). Characteristic tuberculous lesions occur most frequently in the lungs and the retropharyngeal, bronchial and mediastenal lymph nodes. Lesions can also be found in the mesenteric lymph nodes, liver, spleen, on serous membranes, and in other organs (OIE, 2010).
Bovine TB can be transmitted from animals to humans and vice-versa. The most common means of transmission is through the respiratory system. Invisible droplets (aerosols) containing TB bacteria may be exhaled or coughed out by infected animals and then inhaled by susceptible animals or humans. The risk of exposure is greatest in enclosed areas, such as barns. Inhalation of aerosols is the most common route of infection for farm and ranch workers and veterinarians who work with diseased livestock. Livestock also are more likely to infect each other when they share a common watering place contaminated with saliva and other discharges from infected animals. Calves and humans can contract bTB when they drink unpasteurized milk from infected cows (Radostits et al., 2000; Ayele et al., 2004).
Studies carried out in several parts of the world, both in developed and developing countries, identified herd size as one of the major bTB herd-level risk factors (Porphyre et al., 2012). The more cattle there are on a farm, the greater the probability that one of them will acquire the infection. Large herds generally pasture on a larger area, with a higher probability to have more contiguous herds, thus increasing the risk of cattle-to-cattle spread (Reilly and Courtenary, 2013). In addition to that, researchers have explored the breed, pregnancy status, type of farming, parity, and stray animals as risk factors for TB and other contagious diseases of farm animals (Leghari et al., 2016; Mangi et al., 2015; Soomro et al., 2014).
The highest incidence of bTB is generally found in areas where intensive dairy systems are practiced. Dairy production in developed countries follows a trend towards increased intensification on a smaller number of larger production units, which implies increased contact between animals and thus an enhanced risk of bTB transmission. In these intensive systems, aerogenic transmission of bTB seems to dominate (Kaneene et al., 2012). Studies in Ethiopia had reported that the severity of bTB was significantly higher in cattle kept indoors at a higher population density than in cattle kept on pasture. In addition to close contact,the stress caused by overcrowding or nutritional differences between housed and pastured animals was mentioned as contributing to the spread of the disease (Ameni et al, 2006).
Several diagnostic tests are available for diagnosis of bTB in livestock like serological assays, culture test, and comparative intradermal tuberculin (CIDT) test. Among all theseavailable approaches CIDT is known as a gold standard method that used intradermal inoculation of PPD (mammalian purified protein derivative) tuberculin on the neck and 72 hours after the presence of swelling (delayed hypersensitivity) is the indication of infection (Angus, 1978).In Pakistan, previous studies have reported the 0.51-12.7% prevalence of bTB using CIDT, that indicates a huge variation. Moreover, no study is available from Karachi, one of the biggest city of Pakistan. Therefore, the current study was designed to estimate the prevalence of bTB in smallholder dairy farms of Karachi, Pakistan. Moreover, risk factors related to the prevalence of bTB were also investigated.
MATERIAL AND METHODS
Description of the Study Area
A cross-sectional study was conducted to determine the prevalence of bTB and associated risk factors in and around Karachi towns smallholder dairy farms, which is a capital city of Sindh province. Karachi has an arid climate, moderated by the oceanic influence of the Arabian Sea. The city has low annual average precipitation levels (approx. 250 mm per annum). The summers are hot and humid, while the winter climate is dry and lasts between December and February.
Study Population and Analytical Procedures
The city of Karachi consists of eighteen towns, out of which, five peri-urban towns (namely, Bin Qasim, Gaddap, New Karachi, Korangi and Super Highway) with thickly populated dairy farms were selected for CIDT test. Only five union councils from each town were randomly selected and forty suspected animals (both cattle and buffalo) were selected from each union council based onsuspecting criteria that includedifferent clinical signs, i.e., low milk production, weakness, intermittent diarrhea, no response to anthelmintic drugs, irregular febrile and stubborn recurring mastitis. A total of one thousand animals (435 cows and 565 buffaloes) were considered suspected and subjected to CIDT test. The test animal was restrained and two sites, 12 cm to 15 cm apart, on the lateral side of the mid neck were shaved to measure a skin-fold thickness using a caliper. The skin thickness was measured with calipers before the PPD tuberculin was injected. The mammalian purified protein derivative for tubercle bacilli (PPD tuberculin) was obtained from Veterinary Research Institute, Ghazi Road, Lahore, Pakistan. After 72 hours, the thickness of the skin at the injection sites was measured, using digital calipers (Khan and Khan, 2007).
Each smallholder dairy farm owner of the animal was interviewed using a pre- designed questionnaire format. A questionnaire format had been included a semi-structured closed questionnaire on livestock management characteristics, such as herd size, mixing of cattle or cattle contact with other cattle herds, purchasing of animals and housing system especially the sanitation, and ventilation of the house. The dairy animals found in the farms included in the study were noted for the detailed information through recording the age, breed, sex, body condition, reproductive status, origin, and herd size. The dairy animals fit for the study (excluding those calves under six months of age and late pregnancy (>7 months) were subjected to CIDT test.
Data Collection and Analysis
All data recorded were subject to analysis using SPSS 16.0 version. Individual animal level prevalence was computed as the number of positive reactors per 1000 animals tested and the herd level prevalence was calculated as the number of herds with at least one reactor animals per 120 herds. The variations among different factors were analyzed using Pearson’s Chi-square (χ2) test for the occurrence of bTB in animals. In all analyses, confidence level was held at 95% and p-value<0.05 was set for significance at the 5 % significance level.
RESULTS
Prevalence of BTB in Bovine Population of Karachi
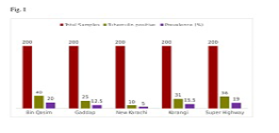
As shown in Figure 1, a total of 1000 suspected animals were screened for bTB through CIDT in peri-urban towns of Karachi. Out of these one thousand clinically suspected animals, 144 were found to be tuberculin positive. The prevalence of tuberculosis was lower in New Karachi (5%) as compared to that of Gaddap (12.5%), followed by Korangi (15.5%), Super Highway (19%) and Bin Qasim (20%). There was a significant difference (p<0.05) in the prevalence of bTB in the bovine population among peri-urban towns of Karachi.
Herd Level Prevalence
A herd level prevalence of 10% (12/120) was recorded by CIDT in and around Karachi towns smallholder dairy farms (Table 1). The differences in prevalence among the different sized herds were statistically significant (χ2= 2.66, p= 0.000), and there was a significant difference in prevalence of bovine tuberculosis with a management system (χ2= 5.16, p= 0.023). However, there was no significant difference associated with the duration of dairy farm establishment (χ2= 4.058, p= 0.131).
Table 1: Prevalence of bovine tuberculosis in bovine population of Karachi in relation to herd level factors.
|
Variables |
Category |
Number of herds tested |
Number of positive herds (%) |
Chi-square |
p- value |
|
Herd size/ number of animals |
<5 |
72 |
0(0) |
2.66 |
0.000 |
|
5-10 |
40 |
8(20) |
|||
|
>10 |
08 |
4(50) |
|||
|
Age of farm established (years) |
<5 |
10 |
0(0) |
4.058 |
0.131 |
|
5-10 |
18 |
2(11.11) |
|||
|
>10 |
92 |
10(10.86) |
|||
|
General management |
Poor |
44 |
8(18.2) |
5.16 |
0.023 |
|
Good |
76 |
4(5.3) |
Individual Animal Level Prevalence
As shown in Table 2, during present study, the individual animal level prevalence of 14.4% (144/1000) was recorded. Thereby the prevalence of bTB was varied significantly among different age groups (χ2=81.14, p=0.000). The difference in reactivity to CIDT test in relation to various physical conditions and reproductive status of female animals were also statistically significant (χ2=18.74, p= 0.001 and χ2=26.63, p= 0.009 respectively). Likewise,associations of breed and sex of animal with a prevalence of bTB were statistically significant (χ2=3.83, p=0.003 and χ2=1.101,p = 0.005 respectively). Origin of the animal also has a statistically significant association with the prevalence of bTB (χ2=8.27, p= 0.008).
DISCUSSION
Pakistan is one of the 22 countries accounting for 80% of total TB infection globally and is one of the 5 countries responsible for half of the TB globally (Metzger et al., 2010b). While a lot of effort are needed to progress the detection rate, that is about 27% (WHO, 2010), Currently, TB is usually diagnosed by a skin tuberculin test that includes a caudal fold, comparative cervical, single cervical, or double-strength cervical test. It has very low sensitivity ranges from 65-70% (Morris et al., 1994). Whereas, in some cases,skin tests have a high specificity about 98% (Morris et al., 1994). False-positive results may occur due to exposure to atypical Mycobacteria, Corynebacteria, Fasciola hepatica, and/or Nocardia species that are problematic in many Asian countries (Peter et al., 2015).In current study, we also prefer the tuberculin test for present study because of its good specificity and economical as compared to other costly methods like ELISA or PCR.
Table 2: Prevalence of bovine tuberculosis in bovine population of Karachi in relation to various risk factors
|
Variables |
Category |
No of animals tested |
No. of positive animals (%) |
Chi-square |
p- value |
|
Age (years) |
≤2 |
326 |
10(3.07%) |
81.14 |
0.000 |
|
>2<5 |
428 |
25(5.84%) |
|||
|
≥5≤7 |
150 |
43(28.67%) |
|||
|
>7 |
96 |
66(68.75%) |
|||
|
Body condition |
Poor |
278 |
120(43.16%) |
18.74 |
0.001 |
|
Medium |
319 |
18(5.64%) |
|||
|
Good |
403 |
6(1.49%) |
|||
|
Sex |
Male |
314 |
36(11.46%) |
1.101 |
0.005 |
|
Female |
686 |
108(15.74%) |
|||
|
Breed |
Cross |
354 |
106(16.41%) |
3.83 |
0.003 |
|
Local |
646 |
38(10.73%) |
|||
|
Reproductive status |
Lactating |
425 |
10(2.35%) |
26.63 |
0.009 |
|
Dry |
248 |
111(44.76%) |
|||
|
<6 month pregnant |
30 |
13(43.33%) |
|||
|
Calf |
225 |
4(1.78%) |
|||
|
Heifer |
72 |
6(8.33%) |
|||
|
Origin |
Purchased |
700 |
75(10.71%) |
8.27 |
0.008 |
|
Born |
300 |
69(23.0%) |
The present study was designed for surveillance of tuberculosis in the bovine population of Karachi, Pakistan. The prevalence of individual animal level (14.4%) found in the present study was higher than reported from Tanzania i.e., 1.3% (Shirima et al. 2003) and Ethiopia i.e., 0.9% (Tschopp et al. 2010).The prevalence shown in this study was still lower as compared to the previous result reported from central Ethiopia, where 7.9% prevalence was observed (Ameni et al., 2003).Whereas, (Asseged et al., 2004) recorded a similar individual animal level prevalence in Addis Ababa and this suggested that bTB epidemiology in smallholder dairy farms could be varied in different areas. This might be due tonumerous husbandry practices and increased number of crossbreds as well as exotic breeds in the dairy farms (Mangi et al., 2015).Our results of all five peri-urban areas are also indicating the area-wise differences in the prevalence rate of bTB (5-20%, Figure 1).
In contrast to the previous report by (Asseged et al., 2004), the significant difference between different body conditioned cattle occurred in this study are in agreement with results of (Ameni et al., 2001). This may be due to the fact that bovine tuberculosis is common and very serious in poor body conditioned animals with the established fact that poor nutrition predisposes to tubercle infection and higher prevalence of zoonoticdiseases is expected in animals with an unhealthy body condition (Yousaf et al., 2016).
Many reports indicated that when herd size is increased, the risk of cattle within the herd presenting a positive reaction also increased (Asseged et al., 2004). During the present study, skin tuberculin test results revealed a significant difference (p< 0.05) with herd size. Results are consistent with previous reports (Cook et al., 2008), and may arise from the fact that increased contact with larger herds favors the lateral spread of infection within a herd, making the prevalence of infection greater in larger herds as shown in Table 1. This occurred as earlier described by (Radostits et al., 2000) who reported that the bTB is an overcrowding disease. Thus, when the number of cattle is increased, the transmission of the disease is accelerated in the herd.Similarly,in this study,CIDT result indicates a statistically significant association with herd management conditions, suggesting the risk of tuberculosis is increased due to poor managerial inputs (Ameni et al., 2003). Earlier studies had also shown higher infection rates in the farms due to poor management conditions (Elias et al., 2008).In this study, the proportion of reactors observed were increased with age. Because, it is well established that a young animal might be exposed to the pathogen, but express the disease in adult age (Griffin et al., 1996).
Few studies reported that pregnant cattle showed lower reactivity due to stress induced immuno-suppression (Leghari et al., 2016; Wood et al., 1991) which is in accordance with the findings of the present study. This might be due to the loss of the sensitivity to tuberculin shortly before and after calving (Radostits et al., 2000).In this study, there is a significant difference in the origin of the animal (p<0.05). Because, it is well established that, purchase of animals is highly associated with bTB positivity (Tschopp et al., 2010).
Breed-based analysis indicated that prevalence of bTB was statistically (p<0.05) higher in cross breed cattle than that of indigenous cattle (Table 2). When compared with previous reports, the results of the current study were consistent with that of (Ameni et al., 2003), but contrary with the results reported by (Alemayehu, 2014). This is most obvious in areas where exotic cattle are used to establish a dairy industry, as exotic dairy breeds (that usually used for crossing) are also less resistant to bTB than the indigenous/local cattle breeds (Leghari et al., 2016).
In harmony with (Cleaveland et al., 2007; Gumi et al., 2012) from Tanzania and southern Ethiopia, results of this study showeda significant positive association between sex and intradermal skin (Table 2). Some other studies also reported positive association between sex and intradermal skin e.g., (Inangolet et al., 2008) testified that female cattle are at greater risk for being positive in Nigeria than males, while (Kazwala et al., 2001) reported that the male cattle were more affected than female as male cattle are castrated and are kept for longer time and so there are more chances of getting infected.
In summary, bTB is present in significant proportions in bovine population in peri-urban areas of Karachi. Old aged animals with the poor physical condition were more prone to the bTB as compared to young animals with good health. Moreover, large herd size, poor management condition, female sex, cross breed, purchased and dry status of animals also contribute to the disease prevalence to a considerable proportion.
ACKNOWLEDGEMENTS
Authors are highly thankful to farmers for their help and cooperation during sample collection.
CONFLICT OF INTEREST
The authors declare no any conflict of interest.
AUTHORS’ CONTRIBUTION
This study was a part of PhD work of M.R.Memon. A.L. Bhutto, P. Khatri and M.G. Shah were the mentor of the project, while M.I. Memon help in preparation of this manuscript.
REFERENCES