Journal of Animal Health and Production
Research Article
Monitoring the Hemato-Biochemical Values in Clinical Cases of Cattle With Respiratory Disorders
Heba El-Zahar*, Heba Gouda, Abbas El-Naggar, Aziza Eissa, Shimaa Gouda
Internal Medicine, Department of Animal Medicine, Faculty of Veterinary Medicine, Zagazig University, Egypt.
Abstract | Respiratory disorders have an economic effect on the dairy industry. The purpose of this study was to diagnose cattle with respiratory problems, as well as to assess the nature of the lesions. A variety of practical approaches are used to examine respiratory disorders in cattle. The present study involved 55 cows of which 10 cows were clinically healthy and served as the control group. Cows were brought to the clinic for a number of complaints, including inappetence, cough, dyspnea and nasal discharges. The total leucocytic counts, aspartate aminotransferase activities, and carbon dioxide tension were significantly higher in all diseased cows. The ultrasonographical examination of the lungs demonstrated several comet-tail artifacts in cows affected with pulmonary emphysema. Various degrees of pulmonary consolidation in bronchopneumonic cows were observed. Furthermore, cows with pleurisy showed anechoic pleural effusion with fibrin deposits and an irregular pleural surface. It is concluded that, hematological and biochemical findings are the key indicators of bovine health, and they are more expressive when correlated with the history, clinical symptoms, and ultrasonographic examination of cows with respiratory diseases.
Keywords | Hematology, Biochemistry, Ultrasonography, Cattle, Respiratory diseases
Received | July 21, 2021; Accepted | August 08, 2021; Published | October 01, 2021
*Correspondence | Heba El-Zahar, Internal Medicine, Department of Animal Medicine, Faculty of Veterinary Medicine, Zagazig University, Egypt; Email: hebaelzahar@gmail.com
Citation | El-Zahar H, Gouda H, El-Naggar A, Eissa A, Gouda S (2021). Monitoring the hemato-biochemical values in clinical cases of cattle with respiratory disorders. J. Anim. Health Prod. 9(4): 391-397.
DOI | http://dx.doi.org/10.17582/journal.jahp/2021/9.4.391.397
ISSN | 2308-2801
Copyright © 2021 El-Zahar et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Respiratory diseases of cattle are caused by infection of the lungs by viruses, bacteria, and/or mycoplasma. Bad management is a predisposing factor (Dubrovsky et al., 2019). Cattle are most susceptible to pulmonary lesions compared to other species, and this is attributed to the anatomy of the bovine respiratory system (Cooper and Brodersen, 2010). Bovine lungs have low gaseous exchange ability and a higher basal ventilation activity, which predisposes them to low oxygen levels during periods of metabolic activity (Flock, 2004; Hussein et al., 2018), which is mostly observed during housing periods. Bovine respiratory disease is not observed in early stages, although it spreads rapidly within the herd (Gorden and Plummer, 2010). Bovine respiratory disease can be treated by the administration of anti-inflammatory drugs and the appropriate antibiotics to minimize the risk of spread of the disease. The medical costs for bovine respiratory disease treatment and the effect on meat quality result in elevated economic costs (Brooks et al., 2011). In addition, bovine respiratory disorders result in reduced fertility in adult cattle, which leads to economic losses. The respiratory diseases in cattle can be assessed by various methods, including lung auscultation, percussion, blood ancillary tests, thoracic radiography, and ultrasonography, in addition to pulmonary aspirations and biopsies (Scott, 2013). In veterinary medicine, ultrasonography of the chest is used for the diagnosis of pulmonary, pleural, and heart diseases. Ultrasonography is a non-invasive technique widely used for the diagnosis of heart diseases (Babkine and Blond, 2009; Flock, 2004; Tharwat and Oikawa, 2011). The aim of the present study was to compare the results of lung ultrasonography in cows with respiratory disorders with the results of hematological and biochemical analysis and correlating it with the history and clinical symptoms.
Materials and Methods
Animals and study design
Fifty five cows aged between 1 and 1.7 years were included in the present study. Forty five cows were examined at the Animal medicine department, Faculty of Veterinary medicine, Zagazig University, Egypt. The examined cows were admitted with a history of respiratory tract disease and presented with a variety of clinical signs, including inappetence, decreased body weight, cough, dyspnea, and variable degrees of nasal discharge. On admission to the animal hospital, a complete, thorough clinical examination was performed (Jackson and Cockcroft, 2002), which included general behavior and condition, auscultation of the lungs, heart, rumen, and intestine. Furthermore, rectal temperature, heart rate, and respiratory rate were recorded. The duration of the illness, based on the owners’ complaints, ranged from 1 to 8 weeks. After sampling collection, the animals were subjected to trial for treatment at the clinic. The remaining 10 dairy Friesian cows owned by the clinic were included in the present study as a control. The control group was apparently normal and on physical examination it was normal. It has no clinical signs or a history of previous respiratory illness.
Blood samples collection and analysis
Two blood samples were collected from the jugular vein, the first placed in an EDTA tube for whole blood collection and the second in a plain tube for serum collection. The first blood sample was used for hematological analysis, including packed cell volume, hemoglobin, erythrocytic, leucocytic and differential counts were performed using an automated cell counter (HA-Vet Hematology Analyzer®, Clindiag Systems B.V.B.A, Belgium). The second blood samples were centrifuged for serum collection. Serum samples were then frozen for later analysis. In serum samples, the concentrations of total protein, albumin, blood lactate, sodium, potassium, and calcium were measured using commercial test kits. Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) activities were measured. The biochemical analysis was performed spectrophotometrically using test kits (Spinreact®, Spain). An arterial blood sample was collected using heparinized syringes for measuring blood gases; a blood gas analyzer (ST-200 CC, Blood Gas Analyzer®, Sensacore, India) was used.
Thoracic ultrasonography
The ultrasonographic examination was done according to previously described studies. The area of ultrasonographic examination extends from 3rd to 11th intercostal spaces (ICS) was prepared by clipping, shaving, and cleaning with alcohol, and then ultrasonographic gel was used. Lungs were examined from both sides according to a standard examination technique. Ultrasonography was performed with a 3.5 - 5 MHz convex transducer (WED, WELLD®, Shenzhen Well D Medical Electronics Co., Ltd., China). Lungs were examined from a dorso-ventral plane by holding the transducer parallel to the ribs. The normal lung appears on ultrasonography as reverberation artifacts, which appear as echogenic lines that run parallel to each other. Abnormal lungs during ultrasonography examination showed the presence of anechoic pleural fluids, absence of reverberation artifacts in lung consolidation, comet-tail artifacts and pleural thickening and irregularity (Babkine and Blond, 2009; Tharwat and Oikawa, 2011).
Statistical analysis
The statistical program SPSS (Version 17.0 released in 2008, SPSS Inc., Chicago) was used. The data was normally distributed according to the Shapiro Wilks W Test. A Paired sample t-test was used to compare the differences between the hematological and biochemical results. The Chi-Square Test was used to compare the categorical variables (cough, auscultation, and percussion results) in association with the health status of cows (healthy or diseased cows). The significance level was set at probability levels of P<0.05, P<0.01, and P<0.001. All data was listed as mean ± SE.
Results
In the present study, a total of 55 cows were included. Forty five cows aged between 1 and 1.7 years were examined at the Animal medicine department, Faculty of Veterinary medicine, Zagazig University, Egypt. After performing a complete and thorough clinical examination, cattle with respiratory disease had a mean body temperature of 39.9°C, a mean pulse of 95 beats per minute and a mean respiratory rate of 47 breaths per minute. Based on the clinical examination, there were 22 animals that exhibited depressed behavior and variable degrees of nasal discharge, which ranged from serous to muco-purulent in consistency. Thirteen cows (28.9 %) showed cough after induction and 27 cows (62.2 %) coughed spontaneously. 23 diseased cows showed dyspnea, 10 cows (22.2 %) with increased vesicular sound, 9 cows (20 %) had a pleural friction sound, and 6 cows (13.3 %) had wheezes sound and 9 cows (20 %) showed no sounds on auscultation. The percussion of the lung area revealed decreased resonance in 25 cows (55.6 %) and increased resonance in 9 cows (20 %). The control
Table 1: Clinical signs and physical examination findings in cows with respiratory disease (Diseased group, n=45) and healthy cows (control group, n=10). Results were expressed as mean ± SE.
| Clinical signs and physical examination findings | Control group (n=10) | Diseased group (n=45) | Sig. |
| General systemic statement | |||
| - Internal body temperature °C | 38.5 ± 0.4 | 39.9 ± 0.6 | |
| - Pulse Rate/ minute | 65 ± 4.6 | 95 ± 5.1 | |
| - Respiratory Rate/ minute | 25 ± 3.4 | 47 ± 2.1 | |
| Coughing | |||
| - Coughing up on stimulation | n=0 (0 %) | n=13 (28.9 %) | 0.000 |
| - Spontaneous coughing | n=0 (0 %) | n=27 (62.2 %) | |
| Percussion of lung | |||
| - Reduced resonance | n=0 (0 %) | n=25 (55.6 %) | 0.000 |
| - Increased resonance | n=0 (0 %) | n=9 (20 %) | |
| Auscultation of the lungs | |||
| - Exaggerated vesicular sound | n=0 (0 %) | n=10 (22.2 %) | 0.000 |
| - Wheezing sound | n=0 (0 %) | n=6 (13.3 %) | |
| - Absence of lung sounds | n=0 (0 %) | n=9 (20 %) | |
| - Pleuritic frictional sounds | n=0 (0 %) | n=9 (20 %) | |
Table 2: Hematological and biochemical findings in control cows and in cows with respiratory diseases. Results were expressed as mean ± SE; the significance level was set at * P<0.05, ** P<0.01 and *** P<0.001.
| Parameters | Control group (n = 10) | Diseased group (n = 45) | Reference values (Constable et al., 2017) |
| - pH | 7.45 ± 0.03 |
7.36 ± 0.02** |
7.35 - 7.50 |
|
- pCo2 |
33.86 ± 1.46 |
43.6 ± 2.02*** |
20 - 30 |
|
- pO2 |
22.16 ± 1.10 | 21.82 ± 1.2 | 34 - 45 |
|
- HCO3 |
26.56 ± 1.55 |
32.14 ± 0.89** |
20 - 30 |
| - BE (mmol/L) | 2.5 ± 0.52 |
5.04 ± 0.55** |
2 - 2.5 |
| - Hb (g/dL) | 11.5 ± 0.6 |
9.66 ± 0.52* |
8.0 - 15.0 |
| - PCV (%) | 28.36 ± 1.02 |
31.66 ± 1.41* |
24 - 46 |
|
- Erythrocyte count (×106/mL) |
6.84 ± 0.31 |
5.51 ± 0.50** |
6.1 - 10.6 |
|
- Leucocytic count (×103/mL) |
6.44 ± 0.71 |
13.31 ± 1.05*** |
6.3 - 14.4 |
| - Neutrophils (%) | 29.06 ± 1.40 |
47.89 ± 3.26*** |
9 - 46 |
| - Eosinophils (%) | 2.55 ± 0.39 |
0.98 ± 0.27** |
0 - 21 |
| - Lymphocytes (%) | 46.32 ± 2.16 |
27.86 ± 2.96*** |
43 - 83 |
| - Total protein (g/dL) | 7.08 ± 0.16 |
5.42 ± 0.76** |
5.7 - 8.1 |
| - Albumin (g/dL) | 3.43 ± 0.42 |
1.85 ± 0.52* |
2.1 - 3.6 |
| - Globulin (g/dL) | 3.65 ± 0.52 | 3.87 ± 0.29 | 0.75 - 2.27 |
| - AST (u/L) | 93 ± 2.02 |
178.7 ± 23.68*** |
43 - 127 |
| - ALT (u/L) | 31.04 ± 0.98 | 30.64 ± 1.11 | 29 - 32 |
| - Blood lactate (mmol/L) | 0.81 ± 0.07 |
2.55 ± 0.38*** |
0.42 - 0.74 |
| - Na (mmol/L) | 130.96 ± 0.64 |
134.25 ± 0.84*** |
132 - 152 |
| - K (mmol/L) | 3.48 ± 0.68 |
5.87 ± 0.21*** |
3.9 - 5.8 |
| - Ca (mmol/L) | 2.19 ± 0.18 |
1.24 ± 0.43** |
2.43 - 3.1 |
cows (n=10) showed no signs of respiratory disease and the physical examination was normal. The mean body temperature was 38.5°C, the mean pulse was 65 beats per minute, and the mean respiratory rate was 25 breaths per minute (Table 1).
Hematological and biochemical findings
Table (2) summarizes the hematological and biochemical measurements in cows with respiratory disease. The arterial blood gas analysis showed a highly significant increase (P<0.001) in pCO2 in the diseased group (43.6±2.02) compared to the control group (33.86±1.46). In addition, there was a moderate significant increase (P<0.01) in pH (7.36±0.02), HCO3 (32.14±0.89) and a mild significant increase (P<0.05) in Hb (9.66±0.52) and PCV (31.66±1.41). Complete blood count findings showed a highly significant increase in the total white blood cell count (13.31±1.05), neutrophils (47.89±3.26) and a moderately significant increase in red blood cell count (5.51±0.50) compared to the control group. The serum activities of AST demonstrated a highly significant increase (178.7±23.68) where there was no significant change in serum ALT activity compared to the control group. In addition, the mean values of serum total proteins (5.42±0.76), albumin (1.85±0.52), sodium (134.25±0.84), potassium (5.87±0.21), calcium ions (1.24±0.43) and blood lactate (2.55±0.38) were significantly increased in cows with respiratory disease.
Ultrasonographic findings
The ultrasonographic examination of the lungs in the healthy control group showed the pleural layers as long, smooth, white linear echo lines that move synchronously with respiration. The normal lung parenchyma was imaged as reverberation artifacts that appear regularly and parallel to the pleura owing to the presence of air (Fig. 1).
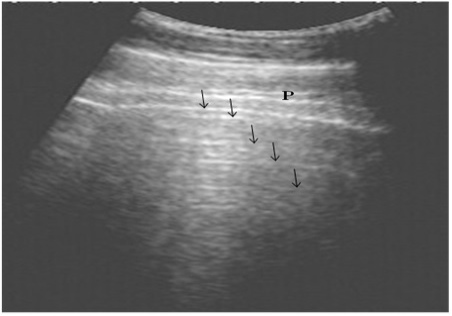
Figure 1: Ultrasonography of the normal lung in healthy cows using a 3.5 MHz transducer. The pleural layers appear as white linear echogenic lines. Reverberation artifacts appear as echogenic lines parallel to the pulmonary surface (Arrows). P is the parietal and visceral pleura.
Nine cattle suffered from pulmonary emphysema showed multiple comet-tail artifacts in the form of bright, closely echo bands beginning at the surface of the lung and run
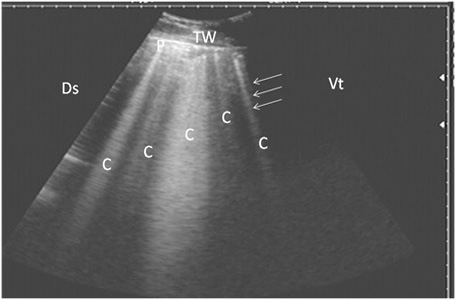
Figure 2: Ultrasonography of pulmonary emphysema in 9 cows using a 3.5 MHz transducer. The image shows multiple comet-tail artifacts in the form of bright echogenic bands (white arrows, C), pleura (P) and the thoracic wall (TW). The right part of the image is ventral (Vt) and the left is dorsal (Ds).
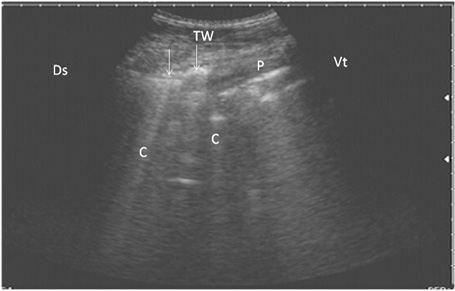
Figure 3: Ultrasonography of bronchopneumonia in cows using a 3.5 MHz transducer. The image shows small hypoechoic circular zones at the lung surface with a comet-tail artifact (white arrows, C), pleura (P) and the thoracic wall (TW). The right part of the image is ventral (Vt) and the left is dorsal (Ds).
ning perpendicular to the pleura (Fig. 2). Twenty-seven cows suffered from bronchopneumonia showed small hypoechoic circular zones on the lung surface with a comet-tail artifact on ultrasonographic examination. In severe pneumonia with lung consolidation, reverberation artifacts were ill defined and unclear (Fig. 3). In 5 cows with pleurisy, the ultrasonographic examination showed echogenic fluid in the pleural sac with the presence of fibrin shreds and the pleural appeared thick and corrugated (Fig. 4). In four cases, moderate pleural effusion revealed hypoechoic fluid and echogenic bands in the pleural sac with comet-tail artifacts (Fig. 5).
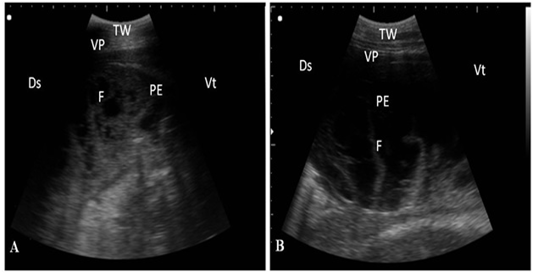
Figure 4: Ultrasonography of pleurisy in cows using a 3.5 MHz transducer. (A) The image shows the presence of thick fragmented visceral pleura and pleural sac filled with echogenic fibrin shreds and anechoic exudates. (B) The image shows pleural effusion and echogenic fibrin shreds. Visceral pleura (VP), fibrin (F), pleural effusion (PE), and the thoracic wall (TW). The right part of the image is ventral (Vt) and the left is dorsal (Ds).
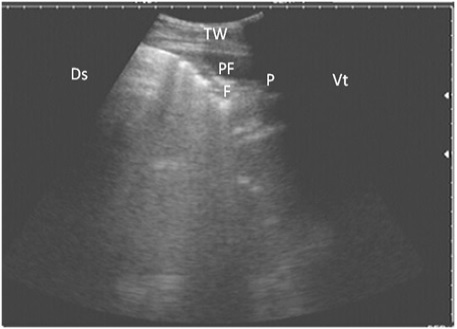
Figure 5: Ultrasonography of the lung of a diseased cow using a 3.5 MHz transducer. The image shows hypoechoic fluid in the pleural cavity with echogenic bands that were hyperechoic with comet-tail artifacts. Pleura (P), fibrin (F), pleural effusion (PE), and the thoracic wall (TW). The right part of the image is ventral (Vt) and the left is dorsal (Ds).
Discussion
The clinical findings that appeared in diseased cows included cough, dyspnea, depression, nasal discharge which ranged from serous to muco-purulent in consistency, and abnormal respiratory sounds. In the present study, cattle with respiratory disease showed increased body temperature, indicating an acute stage of respiratory disease. Fever more than 39.7 °C was set as the selection criteria for cows with respiratory disease as previously described by Scott (2013) and Venkatesakumar et al. (2020).
As a gold standard, blood gas analysis allows for the qualitative and quantitative evaluation of metabolic and respiratory acid-base disturbances, as well as the interrelationship between breathing, oxygenation, and metabolic state. The blood gas analysis showed an increase in the pCO2 and a decrease in the pO2 in diseased cows compared to clinically healthy ones. This suggests impairment of lung function which results in a moderate drop in blood pH, causing respiratory acidosis, and a moderate rise in HCO3 due to compensated metabolic alkalosis and this was in agreement with Hussein et al. (2018). In addition, these results were in agreement with a previous study performed by Carlson and Bruss (2008) who observed changes in pCO2 and pO2 and attributed these changes to decreased alveolar ventilation. Another study found that cattle with respiratory disorders had no changes in the arterial blood gases and they attributed this to the severity of the disease (Tharwat and Oikawa, 2011). There was also a significant rise in blood lactate levels, which might be attributed to decrease oxygen perfusion to tissues resulted in lactic acidosis and metabolic abnormalities (Zeineldin et al., 2017).
Hematological analysis in the present study showed elevation of the leucocytic count and neutrophil percentage in all disease groups. Furthermore, neutrophilic leukocytosis has been linked to a variety of respiratory problems, suggesting active inflammatory reactions in the lungs. The present study was in agreement with Šoltésová et al. (2015) who reported that many illnesses, such as respiratory diseases, may show marked leukocytosis attributed to the infection as a result of inflammatory processes.
Hypoproteinemia and hypoalbuminemia could be attributed to hepatic dysfunction and this was previously described by Constable et al. (2017). Meanwhile, increased activity of AST might result from the increase in the respiration rate and increased movement of the intercostal muscles during the disease periods, this was previously reported by Abdullah et al. (2013) and Hussein et al. (2018).
Ultrasonographic examination of normal lung tissue of healthy cows showed reverberation artifacts that are attributed to the presence of air in the alveoli which reflects the ultrasound echos. The pleura appear as a hyperchoic line. In cows suffering from pulmonary emphysema, there were comet-tail artifacts on the pleural surface and downward, this was in agreement with Hussein et al. (2018). In cows with bronchopneumonia, the pulmonary consolidations appeared on ultrasonographic examination as hypoechoic lung tissues resembling liver tissue (Ollivett and Buczinski, 2016). In cows suffering from pleurisy, the ultrasonography of the pleura revealed thickening and corrugation of the pleural surface with the presence of echoic pleural effusion. As previously discussed (Tharwat and Oikawa, 2011), ultrasonography allows measurement of the pleura, visualization of pleural effusion, and quantification of the nature and duration of the effusion. Finally, ultrasonography of the lung is useful for assessing clinically unclear respiratory problems in animals, and it is an important part of recent bovine medicine (Timsit et al., 2019). The extent and severity of the lung disease in cattle could not be confirmed with clinical certainty, while ultrasonography is a useful method for diagnosing a variety of respiratory problems as well as determining the severity of pulmonary lesions.
It is concluded that, hematological and biochemical findings are the key indicators of bovine health, and they are more closely correlated with the history, clinical symptoms, and ultrasonographic examination of cows with respiratory diseases. Ultrasonography, despite the fact that it is a relatively new clinical tool for diagnosing respiratory disorders, was utilized to confirm the degree, extent and severity of lung abnormalities in diseased cows. Finally, the hematological and biochemical findings in parallel with the history, clinical symptoms, and ultrasonographic examination are of great importance for the diagnosis of respiratory diseases in cows.
Acknowledgements
We would like to express our appreciation to the staff members of the Internal Medicine, Animal Medicine Department, Faculty of Veterinary Medicine, Zagazig University, Egypt for their support and help during the present study.
Conflict of interest
We declare that there is no conflict of interest
authors contribution
All authors contributed equally to this work, they designed the study, collected the samples. Dr. Heba El-Zahar, analyzed the data and wrote the manuscript. All the authors have read and approved the final draft of the manuscript.
References





