Journal of Animal Health and Production
Research Article
Prevalence and Antimicrobial Susceptibility Profiling of Salmonella Isolated from Poultry Products Sold in Sokoto Metropolis, Nigeria
Aliyu Ibrahim Musawa1, Garba Bashiru1*, Agharid Al-Rasheed4, Yusuf Yakubu1, Abdurrahman Hassan Jibril1, Fatima Muhammad Ballah1, Shehu Sidi2, Nafi’u Lawal3, Jamilu Abubakar Bala5, Mohammed Naji Odhah6, Nasir Muhammad1, Maryam Umar1
1Department of Veterinary Public Health & Preventive Medicine, Faculty of Veterinary Medicine, Usmanu Danfodiyo University, Sokoto. 840212, Sultan Abubakar Road, City Campus Complex, Sokoto State, Nigeria; 2Department of Theriogenology and Animal Production, Faculty of Veterinary Medicine, Usmanu Danfodiyo University, Sokoto. 840212, Sultan Abubakar Road, City Campus Complex, Sokoto State, Nigeria; 3Department of Veterinary Microbiology, Faculty of Veterinary Medicine, Usmanu Danfodiyo University, Sokoto. 840212, Sultan Abubakar Road, City Campus Complex, Sokoto State, Nigeria; 4Department of Microbiology, College of Veterinary Medicine, Tikrit University, Iraq; 5Microbiology Unit, Department of Medical Laboratory Sciences, Bayero University, Kano. P.M.B. 3011, Kano, Kano State, Nigeria; 6Department of Veterinary Clinical Studies, Faculty of Veterinary Medicine, University Malaysia Kelantan (UMK), Kota Bharu, Kelantan, Malaysia.
Abstract | The emergence of multidrug-resistant Salmonella in poultry meat and products presents a serious global public health problem. A cross-sectional study was conducted to investigate the isolation rate of Salmonella species in eggs and chicken meat randomly sampled from some selected retail outlets in Sokoto metropolis, and to determine the antimicrobial resistance pattern of the isolates. Bacteriological culture and biochemical characterization, followed by the antimicrobial susceptibility testing using the Kirby Bauer disk diffusion method were employed. Out of the 300 samples comprising 150 eggs, and 150 chicken meat samples analyzed, 20 (13.3 %) were positive for Salmonella among chicken meat, while 11 (7.33 %) were positive among the egg samples. Based on the sampling locations, the frequency of isolation of Salmonella was highest in Sokoto south and Wamakko areas with 17.5 % each for the chicken meat, while Sokoto south area with 10.0 % had the highest among the egg samples. The results of the antimicrobial susceptibility test showed 15 isolates (75 %) for chicken meat being 93.3 %, 86.7 %, 60.0 % and 60.0 % resistant to penicillin, oxytetracycline, Sulphamethoxazole/trimethoprim, and erythromycin respectively, while all 11 (100 %) isolates from egg swab culture showed resistance to one or more of the antimicrobials tested. However, a high proportion of isolates were susceptible to neomycin (93.3 %). The Salmonella isolates also exhibited multidrug-resistance against four of the antimicrobials tested that included erythromycins, Sulphamethoxazole/trimethoprim, penicillin, and oxytetracycline. It could be suggested that the rational use of antibiotics needs to be adopted in commercial poultry farming system of Sokoto to curtail the spread of these drug-resistant pathogens and its concomitant hazard to human health.
Keywords | Antimicrobial resistance, Foodborne infections, Poultry products, Sokoto, Nigeria, Salmonella
Received | December 29, 2020; Accepted | January 19, 2021; Published | April 15, 2021
*Correspondence | Garba Bashiru. Department of Veterinary Public Health & Preventive Medicine, Faculty of Veterinary Medicine, Usmanu Danfodiyo University, Sokoto. 840212, Sultan Abubakar Road, City Campus Complex, Sokoto State, Nigeria; Email: garba.bashiru@udusok.edu.ng
Citation | Musawa AI, Bashiru G, Al-Rasheed A, Yakubu Y, Jibril AH, Ballah FM, Sidi S, Lawal N, Bala JA, Odhah MN, Muhammad N, Umar M (2021). Prevalence and antimicrobial susceptibility profiling of salmonella isolated from poultry products sold in sokoto metropolis, nigeria. J. Anim. Health Prod. 9(2): 148-155.
DOI | http://dx.doi.org/10.17582/journal.jahp/2021/9.2.148.155
ISSN | 2308-2801
Copyright © 2021 Bashiru et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
A wide range of infectious disease pathogens are responsible for most foodborne diseases, which in most cases animals, are the sources of infections to humans (Thomas et al., 2001). Most worrisome, asymptomatic food animals harboring infection usually serve as source of these pathogens to humans (Sánchez-Vargas et al., 2011). Salmonella, being one of the most notorious and pervasive foodborne zoonotic pathogen, worldwide has the potential to cause significant health and economic burden (Abdulhaleem et al., 2019; Marks et al., 2017; Stanaway et al., 2019). In industrialized countries, Salmonella is mostly associated with the economic and social burden (Tabo et al., 2015). However, in resource-poor nations, the bacteria is the major cause of invasive disease, especially among infants and immunocompromised individuals (Graham et al., 2000; Sánchez-Vargas et al., 2011). Traditionally, infection follows the ingestion of raw or poorly processed food, and this is exacerbated by poor personal hygiene practices (Ashurst et al., 2008; Ngogo et al., 2020).
The most common source of human infection is the consumption of contaminated eggs and poultry meat. Largely because Salmonella can survive in meats and other animal products that are not thoroughly cooked, or not adequately stored thereby making them good vehicles of transmission (Antunes et al., 2016; Judd et al., 2019; Salihu et al., 2013). Poultry species such as chickens and turkeys, as well as pigs, cows, and many other domestic and wild animals, are reported to act as reservoirs of Salmonella infection (Brichta-Harhay et al., 2011; Roth, 2013). Most recently, rearing of poultry with other livestocks was identified as a major risk factor for maintenance and spread of Salmonella serovars between different livestock species (Jibril et al., 2020). Salmonella frequently colonizes poultry species via horizontal and vertical transmission, mostly at the primary production level without any detectable symptoms (Antunes et al., 2016; Hugas & Beloeil, 2014). This persistence in healthy poultry birds is considered a significant risk factor because it permits bacterial organisms to be easily transmitted to humans from table eggs and poultry (Hugas & Beloeil, 2014).
In recent years, the poultry industry in Nigeria has blossomed considerably owing to the global food challenges and the government policy on agricultural productivity, to meet up with local demands (Ankers, 2008; Fagbamila et al., 2017). Despite the vast market and productive potentials of the Nigerian poultry industry, the production output is still inadequate. This can be attributed to many factors, including lack of specialization with modern farming techniques as well as the general low level of biosecurity (Ankers, 2008). However, another very important impediment to poultry production in Nigeria is infectious diseases, with influenza and Salmonellosis being among the most prevalent (Akinyemi, Bamiro, & Coker, 2007; Ganau & Manga, 2019). Driven by the desire to meet up with increasing demand for poultry meat and egg, which constitute the major source of animal protein, farmers have resorted to the indiscriminate use of antibiotics as prophylaxis and growth promotion (Adesokan et al., 2015). Unfortunately, this has led to the emergence of antimicrobial-resistant strains of many pathogens like Salmonella. Antimicrobial resistance is a serious public health issue receiving global attention currently. The fact that these resistant bacteria from animals can transfer their resistance genes to the human bacterial pathogen is equally viewed as an additional health hazard. Therefore, this study was undertaken to determine the prevalence of Salmonella in retail eggs and ready-to-eat chicken meat sold in the Sokoto metropolis, and to determine the antimicrobial susceptibility of the Salmonella isolates.
Study location
This study was carried out in the Sokoto metropolis, Sokoto State, Nigeria. Poultry farming (both commercial and backyard) engaged over 70% of the population in the state. Also, the practice of selling roasted or fried chicken meat operating as commercial outlets in several distributed hubs in the metropolis is predominantly practiced by youths. Six locations where chicken meat is sold at night were identified using a purposive (based on popularity and density of customers) sampling approach. Similarly, 12 retail outlets were randomly identified for the sampling of the fresh eggs displayed for sale.
Sample size determination
The sample size for both chicken meat and egg samples was estimated using the formular adapted by (Thrusfield, 2008) with a reported prevalence of 22% by (Fashae et al., 2010), at 5% desired absolute precision and 95% confidence interval.
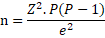
Where n = sample size; P = expected prevalence (11%); Z = 1.96; e = margin of error at 95% confidence interval.
Accordingly, the minimum sample size calculated was 150 for each of chicken meat and egg respectively.
Sample Collection
Between June 2019 and September 2019, 150 fresh chicken eggs and 150 roasted chicken meat sold by the roadside within Sokoto metropolis were sampled. Samples were proportionately allocated to the four local government areas that constitute the metropolis based on the number of selling points; consequently, 40 samples (each of fresh eggs and roasted chicken meat) were allocated to each of Sokoto south, Sokoto north and Wamakko local government areas, while Dange-Shuni local government area was allocated 30 samples each of fresh eggs and roasted chicken meat. All samples collected were transported to the bacterial zoonoses laboratory of the department of Veterinary public health and preventive medicine, Usmanu Danfodiyo University Sokoto for processing and microbial analyses.
Salmonella Isolation
Using sterile swab sticks, the shell of each egg was swabbed into 9 ml of buffered peptone water as diluent and incubated at 37oC for 24 ± 2 hours (Al-Abadi & Mayah, 2012). Similarly, for the chicken meat samples, 1 g of each sample was homogenized in sterile pestle and mortar using 9 ml of buffered peptone water and incubated at 37°C for 24 hours (Kim et al., 2012). For each of the egg and chicken meat samples, 100 µl of the pre-enriched buffered peptone water was transferred into another 10 mL of Rappaport-Vassiliadis (RVS) broth (Oxoid) and incubated at 41°C for 24 hours for selective enrichment. Subsequently, the RVS broth enrichment culture was streaked onto xylose lysine deoxycholate (XLD) agar (Oxoid) and Brilliant Green agar (Oxoid), and the plates were incubated for 24 hours at 37°C. The resultant presumptive Salmonella colonies grown on the XLD and Brilliant green agar (BGA) plates were then sub-cultured on Nutrient agar plates for isolation of distinct colonies.
Biochemical tests
All suspected Salmonella colonies sub-cultured on the nutrient agar plates were inoculated into the following biochemical test tubes for further confirmation: Simon citrate test (positive Salmonella isolates is indicated by the production of intense Prussian bluish colonies denoting alkalinization of the medium on the citrate test) (Al-Dhabaan, 2019), while hydrogen sulfide production, indole, and motility assays were determined using the SIM medium (black precipitate indicate H2S gas production; negative indole test produces no colour change upon the addition of Kovac’s reagent; Motile organism extends from the stab line and produces turbidity or cloudiness throughout the medium) (Salihu et al., 2013).
Antibiotic Susceptibility Test
Antimicrobial susceptibility test was determined according to the Kirby Bauer disc diffusion technique based on the following panel of antimicrobials procured from Oxoid (UK); tetracycline (TE, 30 μg), Sulphamethoxazole/trimethoprim (SXT, 30 μg) neomycin (N, 30 μg), erythromycin (E, 15 μg), and penicillin (P, 10 μg). The antimicrobial susceptibility was deduced by measuring the zone of inhibition. Results were then interpreted as susceptible, intermediate, or resistant according to the Clinical and Laboratory Standards Institute(CLSI) guidelines (Wayne, 2019). Isolates that displayed resistance to more than three classes of antibiotics were described as multidrug-resistant (MDR) as defined by Magiorakos et al. (2012)
Data analysis
The data generated is presented in tables and percentages. Chi square or Fisher’s exact test, as the case may be, was used to determine association between prevalence of Salmonella, sample type and sampling locations using the SPSS statistical software version 23 (IBM Statistics, USA). Values were considered to be statistically significant if p value is less than 0.05 (i.e p<0.05)
Results
Cultural isolation and biochemical characterization
In total, 300 samples comprising 150 fresh chicken eggs and 150 roasted chicken meat collected from different outlets were analyzed for presence of Salmonella. The overall detection rate of Salmonella isolates among 300 samples collected was 10.3 % (CI95 [7.1 – 14.3]) from poultry products in Sokoto metropolis. Based on sample type, roasted chicken meat had the highest detection rate of 13.33 % (20/150) compared to 7.33 % (11/150) obtained from fresh chicken eggs as shown in Table 1. Despite the apparent differences in these prevalence rates, there was no statistically significant difference (p-value = 0.088; X2 = 2.914) between the 2 sample types, however, there seems to be almost twice the risk of acquiring Salmonella from roasted chicken meat than from chicken eggs (OR = 1.944; CI95 [0.9 – 4.2]). Detection rate across the different sampling locations was such that Sokoto south had the highest rate of 13.75 % (11/80), this is followed by Sokoto north and Wamakko each with 11.25 % (9/80). The least detection rate was from Dange-Shuni with 7.33 % (2/60). Again, no statistically significant association (chicken meat [p-value = 0.059; X2 = 7.321]; chicken eggs [p-value = 0.910; X2 = 0.863]) has been found to exist between prevalence of Salmonella (for both roasted chicken meat and chicken eggs) and the sampling location (Tables 2 and 3).
Antimicrobial Susceptibility Profile of the Salmonella Isolates
The result of the antimicrobial susceptibilities of the Salmonella isolates is presented in Tables 4 and 5. From the roasted chicken meat, 15 isolates showed resistance to one or more of the antimicrobials tested with penicillin, oxytetracycline, Sulphamethoxazole/trimethoprim, and erythromycin showing 93.3 %, 86.7 %, 60.0 % and 60.0 % resistance respectively. Notably, a high proportion of isolates were susceptible to neomycin (93.3 %).
Table 1: Prevalence of Salmonella spp. from roasted meat and chicken egg sold within Sokoto metropolis
| Sample type | No. +ve | No. -ve | Prevalence (%) | p-value | X2 | OR | CI |
| Roasted Chicken Meat | 20 | 130 | 13.33 |
0.088 |
2.914 |
1.944 |
0.897 – 4.214 |
| Chicken Egg | 11 | 139 | 7.33 |
OR: Odds ratio; CI: Confidence interval
Table 2: Prevalence of Salmonella from roasted chicken meat across the different sampling locations
| Areas | No. +ve | No. -ve | Prevalence (%) | p-value | X2 | OR | CI |
| Sokoto south | 7 | 33 | 17.5 |
0.059 |
7.321 |
- |
- |
| Sokoto North | 6 | 34 | 15.0 | ||||
| Wamakko | 7 | 33 | 17.5 | ||||
| Dange-Shuni | 0 | 30 | 0 | ||||
| Total | 20 | 130 |
13.3 |
OR: Odds ratio; CI: Confidence interval
Table 3: Prevalence of Salmonella from fresh chicken eggs across the different sampling locations
| Areas | No. +ve | No. -ve | Prevalence (%) | p-value | X2 | OR | CI |
| Sokoto south | 4 | 36 | 10.0 |
0.910 |
0.863 |
- |
- |
| Sokoto North | 3 | 37 | 7.5 | ||||
| Wamakko | 2 | 38 | 5.0 | ||||
| Dange-Shuni | 2 | 38 | 6.6 | ||||
| Total | 11 | 139 |
7.3 |
OR: Odds ratio; CI: Confidence interval
Similarly, 11 (7.3 %) of the isolates from fresh chicken egg swab culture were resistant to one or more than one of the tested antimicrobials. The highest percentage of resistance was 54.5 % for erythromycin, followed by penicillin and oxytetracycline, with 36.4 % each. On the other hand, a large number of the isolates were found to be susceptible to neomycin, Sulphamethoxazole/trimethoprim, oxytetracycline, and penicillin in different proportions (63.6 %, 72.7 %, 63.6 %, and 54.5 %) respectively.
Out of 15 chicken meat and 11 egg swab isolates of the Salmonella, six isolates (40.0 % and 54.6 %) showed multi-drug resistance (resistance to at least three antibiotics). The predominant MDR or drug-resistant patterns among the Salmonella isolates were erythromycins, Sulphamethoxazole/trimethoprim, penicillin, and oxytetracycline.
Table 4: Antimicrobial resistance patterns of Salmonella isolates obtained from chicken meat
| Antibiotics | Breakpoint (mm) | % Resistant | % Susceptible |
| Neomycin | 13-14 | 6 | 93.3 |
| Sulphamethoxazole/trimethoprim | 13-16 | 73.3 | 26.7 |
| Erythromycin | 14-22 | 93.7 | 6.7 |
| Penicillin | 17-18 | 93.3 | 6.7 |
| Oxytetracycline | 14-15 | 86.7 |
13.3 |
Table 5: Antimicrobial resistance patterns of Salmonella isolates obtained from eggs
| Antibiotics | Breakpoint (mm) | % Resistant | % Susceptible |
| Neomycin | 13-14 | 36.4 | 63.6 |
| Sulphamethoxazole/trimethoprim | 13-16 | 27.3 | 72.7 |
| Erythromycin | 14-22 | 90.6 | 9.1 |
| Penicillin V | 17-18 | 45.5 | 54.5 |
| Oxytetracycline | 14-15 | 36.4 | 63.6 |
Discussion
Salmonella is considered one of the most prevalent foodborne pathogens worldwide and constitutes a serious public health hazard following the emergence of drug-resistant strains (Walther et al., 2017). Salmonellosis is usually associated with the consumption of contaminated food prepared under poor hygienic conditions, especially those of animal origin. The disease is endemic in most parts of the world; however, prevalence is higher in resource-limited countries, including Nigeria (Fagbamila et al., 2017; Salihu et al., 2015; Salihu et al., 2013). Human Salmonellosis mostly results from infection with the non-typhoidal Salmonella, which includes; S. enteritidis, S. typhimurium, S. hadar or S. heidelberg (Antunes et al., 2016; Sánchez-Vargas et al., 2011). They mostly invade and colonize poultry without causing any disease or disorders. This investigation was undertaken to determine the presence of Salmonella as a contaminant in poultry eggs and roasted chicken meat sold at retail outlets within the Sokoto metropolis. The overall prevalence based on the cultural isolation and biochemical characterization was found to be 10.3 % (31/300) which is alarming from the public health point of view. Owing to the fact that many residents of Sokoto metropolis and beyond do patronize these poultry products regularly, the dangers of gastrointestinal tract infections are not farfetched. The fact that previous studies by Salihu et al. (2013) and Faleke et al. (2017), both in Sokoto, reported varying prevalence of Salmonella on food contact surfaces indicates a continuing trend in the unhygienic nature with which foods are handled.
From our findings, roasted chicken meat had a 13.33 % (20/150) prevalence while fresh egg swabs had a 7.33 % (11/150) prevalence rate. Although the heat to which the chicken meat is exposed to in the process of roasting is enough to take care of any Salmonella contaminants, the only logical explanation as to why the prevalence is higher in roasted chicken than that on fresh eggshell could be attributed to the unhygienic nature with which the roasted meat is handled. Practices such as the use of unhygienic or dirty utensils (plates, spoons, knives, etc.), using old newspapers or cement packaging papers as wrapping materials, and handling of contaminated paper currency notes with bare hands by the meat seller could lead to contamination of the meat. Also, display of roasted chicken in dirty show glasses, chopping the meat on boards that are constantly hovered by flies or even allowing customers to touch the roasted meat while selecting which to buy can all lead to heavy contamination of the roasted chicken with Salmonella. The habit of garnishing roasted chicken with spices mixed in oils, and the culinary addition of vegetables (onions, tomatoes, cabbage, cucumber, etc.) can all be potential sources of Salmonella cross contamination of the roasted chicken, not to mention the usual habit of allowing one person to be handling both raw and roasted chicken intermittently.
Works on Salmonella contamination of meat and meat products have reported varying prevalence rates in recent times. Rates of 8.2 %, 39 % and 28.8 % have been reported by Salihu et al. (2013), Jajere et al. (2015) and Faleke et al. (2017) respectively, while internationally 5 %, 4.4 %, 6.67 % and 7.1 % have been reported by El-Malek (2017), Khalif et al. (2018), Younis et al. (2019) and Almashhadany (2019) respectively.
On the other hand, many works have also reported varying prevalence rates of Salmonella contamination on eggshells. In Sokoto, a 6.11 % prevalence of Salmonella on the shells of chicken egg was reported by Salihu et al. (2015) and a 9.5 % prevalence of Salmonella on quail egg surface have been reported by Mera et al. (2017), while a 10.3 % prevalence of Salmonella contamination of retail chicken eggs has also been reported in Enugu by Okorie-Kanu et al. (2016). Elsewhere, Zubair et al. (2017) reported a prevalence 4.85 % of Salmonella contamination of chicken shell surfaces in Iraq.
All the similarities or otherwise, between our reported prevalence and those of other authors could be due to geographical location, season of study, sanitary practices and laboratory detection methods.
The antimicrobial susceptibility profile of the isolates was also investigated using panel of antimicrobials (Oxytetracycline, Sulfonamide, Penicillin, erythromycin, and neomycin) where varying levels of susceptibility and resistance were observed. The choice of these drugs was based on their popularity as choice veterinary drugs for the treatment of poultry diseases as well as their frequent use as feed additives (Adesokan et al., 2015; Vermeulen, 2002). The antimicrobial resistance profile for egg isolates revealed that many of the isolates are resistant to erythromycin (54.5 %), while greater susceptibility was recorded for sulfonamides (72.7 %). However, for the ready-to-eat chicken meat, penicillin and oxytetracycline showed the highest resistance with 93.3 % and 86.7 %, respectively. The use of medication at breeder level and repeated use of egg crates and egg storing trays could considerably increase the rate of isolation of multiple resistant Salmonella in poultry as observed in the present study. Although, the majority of the isolates (93.3 %) were susceptible to neomycin, which is one of the drugs commonly prescribed for use for the treatment of poultry diseases. The result of the present investigation is similar to a study conducted on raw chicken meats in Malaysia, where multi-drug resistance was observed among the isolates, including erythromycin (Thung et al., 2018). The detection of drug resistance among the isolates is worrying, in view of the potential transfer of resistance determinants among pathogenic bacteria including human pathogens via horizontal gene transfer (Becker et al., 2018; Timme et al., 2013). Furthermore, the development of resistance to macrolide antibiotics, one of the last resort in treating human Salmonellosis, has been reported to predispose to Streptococcus pyogenes infection which is associated with the majority of cases of sore throat in pediatric patients, as well as other severe life-threatening infections (Efstratiou & Lamagni, 2016; Fyfe et al., 2016; Leclercq & Courvalin, 2002).
Salmonella contamination of eggs and eggshells has been identified as a severe public health concern worldwide. A recent shift in consumer preferences has impacted on the egg industry, with a push for cage-free egg production methods as well as increased desire from consumers for raw and unprocessed foods, thus potentially increasing the risk of salmonellosis (Packierisamy et al., 2018). Similarly, the presence of antimicrobial-resistant Salmonella in the ready-to-eat poultry meat is a potential threat to consumer health, especially individuals with a compromised immune system and those suffering from debilitating illnesses like diabetes.
Conclusion
The high frequency of isolation of drug resistant Salmonella in current study may be attributed to the endemicity of Salmonella spp. in Sokoto potentiated by cross-contamination due to the mixing of cooked and raw food materials in markets and shops. So also, the practice of the open sale of the eggs and meats and frequent contact with hands of potential buyers during examination and choosing may equally be a very important source of the contamination observed in this study, especially because these buyers come from different backgrounds including laborers who work in construction fields, farms and waste disposal workplace.
acknowledgements
We wish to acknowledge the support and cooperation of all the food vendors for letting us collect samples. We also want to acknowledge the support from staff of the bacterial zoonoses laboratory of the Faculty of Veterinary Medicine, Usmanu Danfodiyo University Sokoto.
Conflict of interest
The authors declare no conflict of interest.
authors contribution
AIM, GB, AA, YY, NM & MU designed the work, AIM, GB, AHJ, FMB, SS, NL, JAB, NM & MU participated in sample collection and laboratory analyses; AIM, GB, YY & MNO performed statistical analyses and drafted the manuscript. All authors participated in perfecting the draft manuscript prior to submission.
References





