Journal of Animal Health and Production
Research Article
Detection of Inclusion Body Hepatitis Virus in broilers at Sharkia Province, Egypt
Yara F. El-Basrey1*, Rehab. I Hamed2, Mahmoud Mohamed1,3, Mohamed A Lebdah1
1Department of Avian and Rabbit Medicine, Faculty of Veterinary Medicine, Zagazig University; 2Department of Poultry diseases, Reference Laboratory for Veterinary Quality Control on Poultry Production, Sharkia; 3Department of Clinical Sciences, College of Veterinary Medicine, King Faisal University.
Abstract | During the last 10 years, many inclusion body hepatitis (IBH) outbreaks across Egypt were reported causing high economic losses to poultry industry. The current study aimed the detection and molecular characterization of IBH in broilers at Sharkia governorate. For identification and genotyping of fowl adenoviruses related to IBH in broiler flocks, liver samples representing 40 broiler flocks were collected and tested for FAdVs based on hexon gene detection using polymerase chain reaction (PCR). We recorded most loses among broilers at 3-5 weeks of age. Only depression, ruffled feather were the observed signs with varied mortality (8-14%). On necropsy, liver was yellowish, friable, enlarged and hemorrhagic. For identification and genotyping of Fowl adenoviruses related to inclusion body hepatitis (IBH) in broiler flocks at Sharkia governorate, liver samples representing 40 broiler flocks were collected and tested for FAdVs based on hexon gene detection using polymerase chain reaction (PCR Out of the forty examined broiler flocks, three flocks (7.5%) were recorded PCR positive for Aviadenoviruses. Based on hexon loop-1 gene analysis, the nucleotide sequence identity of the three positive samples (EG101/2018, EG102/2018 and EG103/2018) was >97.7% and genetically was closer to reference strains serotype 2 (FAdV-2), the three examined strains shared a high degree of nucleotide identity (97.6-99.6%). Using neighbor joining for the phylogenetic tree construction depending on hexon gene nucleotides and amino acids sequences, our strains were clustered in the same clade with FAdV type D. This study help to extend the current knowledge about the currently circulating IBH viruses and to develop vaccination and control strategy.
Keywords | Aviadenovirus, Chicken, Inclusion body hepatitis, PCR, Sequencing.
Received | November 03, 2020; Accepted | December 01, 2020; Published | December 27, 2020
*Correspondence | Yara F El-Basrey, Department of Avian and Rabbit Medicine, Faculty of Veterinary Medicine, Zagazig University; Email: yaraelbasrey@yahoo.com
Citation | El-Basrey YF, Hamed RI, Mohamed M, Lebdah MA (2020). Detection of Inclusion Body Hepatitis Virus in broilers at Sharkia Province, Egypt. J. Anim. Health Prod. 9(s1): 84-89.
DOI | http://dx.doi.org/10.17582/journal.jahp/2020/9.s1.84.89
ISSN | 2308-2801
Copyright © 2020 El-Basrey et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Inclusion body hepatitis (IBH) infection in chickens was first described in 1963 in the USA. The susceptibility of the disease in broilers usually is at 21 to 28 days old however, the disease reported in birds aged one week and as older as 20 weeks. Mortality depends on the virulence of the virus, health condition of the bird and coexisting diseases, and may be up to 80% (Howel et al., 1970; Pan et al., 2017).
IBH is associated with fowl aviadenoviruses (FAdVs), which are non-enveloped double stranded DNA viruses with icosahedral symmetry (Zhao et al., 2015; Mase et al., 2009). The virus DNA is associated with many proteins (Ginsberg, 2013), the main proteins are hexon (II) and fibre (IV) with a penton base (III) non-covalently attached, and numerous minor proteins: VI, VIII, IX, IIIa and IVa2 (Russell, 2000).
The existing classification scheme identifies five types of fowl aviadenoviruses A to E (FAdV-A to FAdV-E) on mo
Table 1: Primers sequence and amplicon sizes
| Virus | Sequences of Primer 5’- 3’ | Size | Reference |
| FAdV |
CAA RTT CAG RCA GAC GGT |
890 bp |
|
| TAG TGA TGM CGS GAC ATC AT | |||
| Hexon A |
GTCGCCGTTTTTTTAGGTGGATCCG |
206bp | |
| CTGGATCCAGTTGTTAAAGAAGGCT | |||
| Hexon B |
TGGACATGGGGGCGACCTA |
1219bp
|
|
| AAGGGATTGACGTTGTCCA | |||
| Hexon C |
AACGTCAATCCCTTCAACCACC |
1350bp | |
| TTGCCTGTGGCGAAAGGCG |
lecular basis, whereas twelve serotypes described as FAdV-1-8a & 8b-11 based on serological classification (Harrach et al., 2012). The FAdVs mostly infect numerous species of birds causing many diseases including IBH, bronchitis in quails, gizzard erosion and hepatitis hydropericardium syndrome (HHS) (Steer et al., 2009). Broilers between 15 and 28 days old particularly exposed to IBH, characterized by enlarged, hemorrhagic or congested liver. As characteristic microscopic lesion of FAdVs infection, intranuclear inclusion body commonly reported that usually basophilic and enclosed by a clear halo or occupy the whole nucleus (Nakamura et al., 1999). Moreover, FAdVs may lower the bird’s immunity, accordingly exaggerating the threats of secondary infections (Shamim et al., 2009). Recently, conventional methods of FAdVs characterization have been progressively changed by genomic typing depended on the nucleotide and amino acids sequences of hexon loop-1 (Hex L1) gene (Hess, 2000).
In Egypt, Radwan et al. (2018) isolated serotype 8a Fowl Adenovirus from flocks with age 3-5 week old in Behira Governorate. While, Mohamed and Kamel, (2019) detected 7 positive flocks as species D from 10 flocks during Spring 2017 in Kafr El-Shiekh Governorate. Also, El bestawy et al. (2020) reported serotype 2 and 11 in Northern Egypt during March 2017 to March 2018. The objective of our work was to carryout molecular characterizations and compare FAdVs isolated from actual IBH clinically infected broilers in our locality (Sharkia province) with the FAdVs on Genbank.
Material and methods
Sample collection and processing
All institutional and national guidelines for the care and use of birds were carried out and were approved by the Institutional Animal Care and Use Committee (IACCUC) of Zagazig University.
Liver samples from 40 broiler flocks (5 bird per flock) representing different localities at Sharkia Governorate were submitted to Department of Avian and Rabbit Medicine, Faculty of Veterinary Medicine, Zagazig, Egypt during 2018. The clinical examination was suggestive to IBH as age 20-35 days, mortality between 8-14% with depression and ruffled feather. Post-mortem examination was carried out, samples from liver were collected from freshly dead or euthanized birds.
Liver samples from each flock were pooled (n=5/flock) and grinded in phosphate-buffered saline (PBS, pH 7.0–7.4) with 1:10 w/v ratio. Homogenates were freezed and thawed three times then centrifuged at 3000 × g for 5 min., finally, collection of supernatant for extraction of DNA.
Nucleic Acid Extraction
Extraction of DNA was carried out using DNeasy® Blood and Tissue Mini Kits (QIAGEN, USA) according to instructions of manufacture. Purified DNAs were preserved at -20°C for PCR and Sequencing.
Molecular detection of IBH Virus using PCR
The extracted DNAs were tested to detect FAdVs using HotStartTaq® Plus Master Mix Kit (QIAGEN, USA). 2µl of extracted DNAs was amplified in 20 μl of the final volume of PCR Master Mix containing 1.5 mM MgCl2, 200 μM of each dNTP, 1 unit HotStartTaq Plus DNA polymerase, and 10 μM of each primers set (Table 1). Thermo-cycling profile was at 95°C for 5 min for enzyme activation and initial denaturation followed by 35 cycles at 94°C for 30 sec, 60°C for 30 sec and 72°C for 45 sec and a final extension step at 72°C for 10 min. The amplified PCR products were electrophoresed in 1.2% agarose gel, stained with ethidium bromide and photographed under UV light.
Molecular identification of viral IBH via Gene sequencing:
The entire ORF of Hexon gene was amplified using three pairs of primers that were chosen according to sequence database and analyzed by Oligo Analyze 3.1 IDT Technologies, Germantown, MD, USA. These primers were synthesized by Macrogen, Korea (Table 1).
For entire Hexon gene ORF amplification, the extracted DNA was amplified using HotStartTaq® Plus Master Mix Kit (QIAGEN, USA). 2µl of extracted DNAs was amplified in 20 μl of the final volume of PCR Master Mix containing 1.5 mM MgCl2, 200 μM of each dNTP, 1 unit HotStartTaq Plus DNA polymerase, and 10 μM of each primers set (Table 1). Thermo-cycling profile was at 95°C for 5 min for enzyme activation and initial denaturation followed by 35 cycles at 94°C for 30 s, the annealing temperatures were 61°C, 52°C, 58°C for 30 s (HexA, B,
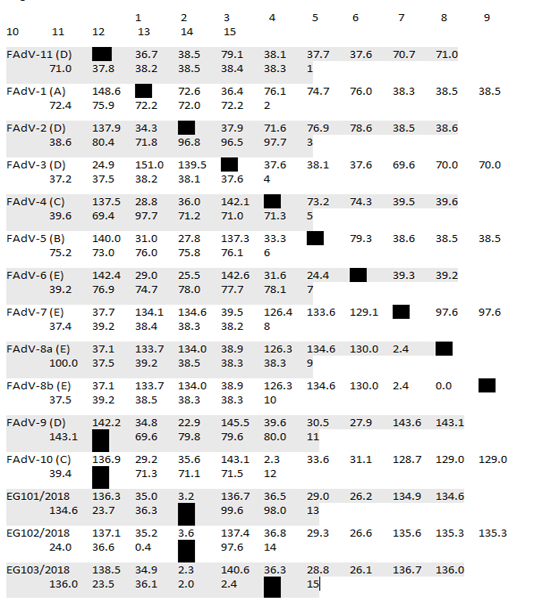
Table 2: Pair Distances of Egyptian isolates with reference strains based on nucleotides sequences alignment
* Divergence in lower triangle
C respectively) and 72°C for 45 sec and a final extension step at 72°C for 10 min. The amplified PCR products were electrophoresed in 1.2% agarose gel stained with ethidium bromide and documented using ultraviolet gel documentation system (BIORAD®, California, USA).
PCR products specific for Hexon gene were purified from the agarose gel using Montàge DNA gel extraction kit (Millipore, USA) and sequenced in an automated ABI 3730 DNA sequencer (Macrogen®, Korea). The sequences were aligned by the Clustal W method and compared with corresponding sequences available in Genbank by BLAST web tool of the Genbank. A phylogenetic tree was made using MEGA version 6.0 software.
Results
External examination and Post-mortem lesions
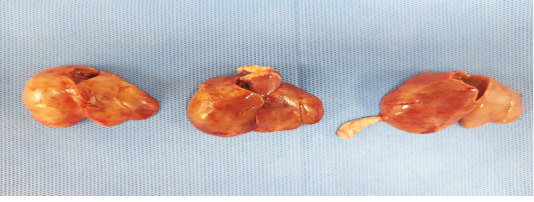
Birds were depressed with mortalities lasts for 3-4 days. Sometimes watery diarrhea. Friable, enlarged liver with echymotic haemorrhage were the most common lesion during necropsy (Figure 1).
Molecular detection of IBHV using PCR
Out of 40 tested clinical liver samples, only three were PCR positive using the screening primer sets targeting the hexon gene producing 890bp band. The sequences of the Hexon gene of the detected FAdVs were submitted to the Genbank database under accession numbers (EG103/2018 MT975970), (EG101/2018 MT975968) and (Eg102/2018 MT975969).
Molecular identification of viral IBH via Gene sequencing and phylogenetic analysis
After assembly, the entire sequences of hexon gene were analyzed to classify the three FAdVs isolates (EG103/2018, EG101/2018 and Eg102/2018). The phylogenetic analysis classifies the three FAdVs in the major clade corresponding to the reference species FAdV-D (2) as shown in Figures 2. The highest nucleotide similarity among the examined strains and reference FAdVs was 97.7% with FAdV-2 (FAdV-D). The divergence between field strains was very low, with sequence similarity ranged from 97.6-99.6% (Table 2). The highest nucleotide similarity between reference strains of the different types was 78.6% between different strains of FAdVs-D and strains belongs to FAdVs-6 (FAdV-E). Moreover, our tested aviadenoviruses showed high similarity and clustered with the Saudi FAdVs when aligned and assembled, Figure (2).
The recently isolated strains (EG/101, EG102 and EG/103) showed high divergence from the FAdVs strains MMR-T1 and MMR-B15 belong to FAdVs subtype E isolated during 2015 from Egypt. The similarity was low, it was varied from 59.9 and 61.5%. The higher percentage of identity was 93.1% between our three isolates and FAdV-D/kfr4 which isolated during 2018 from a geographically related region (Table 3).
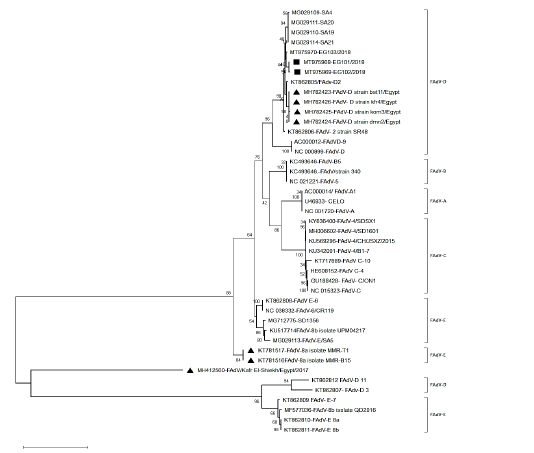
Figure 2: Phylogenetic tree based on the whole sequence of hexon showing three isolates belonged to FAdV-2 (species D). The local isolates labelled with the black square, while other isolates from Egypt labeled with black triangle.
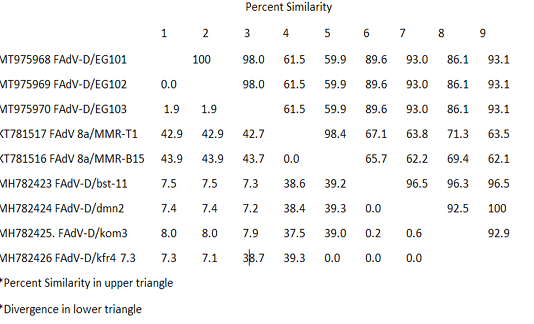
Table 3: Pair Distances of nucleotides sequences of FAdVs isolated from Egypt during the period 2015-2018
Table 4: Reference Fowl adenoviruses (FAdVs) for phylogenetic analysis.
| Genotype | Serotype | Accession number |
| FAdV-A | FAdV-1 | AC_000014 |
| FAdV-B | FAdV-5 | KC493646 |
| FAdV-C | FAdV-4 | HE608152 |
| FAdV-10 | KT717889 | |
|
FAdV-D |
FAdV-2 | KT862805 |
| FAdV-3 | KT862807 | |
| FAdV-9 | AC_000012 | |
| FAdV-11 | KT862812 | |
| FAdV-E | FAdV-6 | KT862808 |
| FAdV-7 | KT862809 | |
| FAdV-8a | KT862810 | |
| FAdV-8b | KT862811 |
Discussion
Recently, fowl aviadenoviruses are reported worldwide in many avian species including wild birds and domestic poultry (Hess, 2013; Mohamed et al., 2018). In Egypt, many authors suggested its incrimination in losses among broilers and more investigation become of necessary to highlight the role of the disease (Radwan et al., 2018; El bestawy et al., 2020). Clinically the diseases was first reported in Mainland China in broilers of three to five weeks age, and occasionally in eggs producing flocks layers) aged ten to twenty weeks of age and always described as HHS (Zhao et al., 2015). During the last 10 years, IBH outbreaks with multiple serotypes occurred all over the world (Schachner et al., 2016, 2017).
In our research, we demonstrated IBH related FAdVs in clinically infected birds exhibited the typical signs and lesions of IBH infections in chickens (Shane, 2000; Chandra et al., 2000; Laanani et al., 2015). Three out of 40 tested samples were positive for Adenovirus serotypes using primers that target Hexon gene. Elbestawy et al. (2020) while working on 37 broiler flocks reported 17 as positive. Radwan et al. (2018) reported that two out of nine commercial broiler flocks (3–5 weeks old) were positive. Mittal et al. (2013) stated a positive relation between number of outbreaks and immune suppressive diseases.
In our study, three strains were detected in chickens with IBH infection, and the entire hexon gene; the major capsid protein and has groups and sub-group determinants (Harrach et al., 2012; Schachner et al., 2016) were sequenced and analyzed. In addition, neighbor joining based phylogenetic tree was constructed using assemble sequences from reference FAdV isolates representing different geographical localities, the analysis showed that the 3 isolates (EG101/2018, EG102/2018 and EG103/2018) were clustered into serotype-2, FAdV-D species.
The entire gene sequences of hexon gene were analyzed. The results revealed very low divergence among the 3 strains ranged from 0.4 to 2.6%. Marek et al. (2013) stated a closer genomic relationship among strains representing the same serotype. The highest nucleotide similarity among the tested and reference strains was 97.7% with FAdV-2 (FAdV-D). Elbestawy and collegue, (2020) characterized 17 strains isolated from different localities in Egypt as fowl adenoviruses D. The study also indicated that the virus is still circulating in the Egyptian poultry flocks and may causes huge losses.
The studied strains (EG/101, EG102 and EG/103) showed high divergence from the FAdVs strains MMR-T1 and MMR-B15 belong to FAdVs subtype E, isolated during 2015 from Egypt. The similarity was low, it was varied from 59.9 and 61.5%. The higher percentage of identity was 93.1% between our three isolates and FAdV-D/kfr4, which isolated during 2018 from a geographically related region. It indicating, the emergence of species D to Egypt in last two years.
Interestingly, the studied strains showed high similarity with very low distance with Saudi strains. The three isolates were clustered with FAdVs strains isolated from Europe, Australia, India and Saudi Arabia in the same clade; this increase the proposition that variances in hexon gene are serotypes related and not locality related (Schachner et al., 2016).
Other fowl adenoviruses type E and type D also reported in Egypt by Radwan et al. (2018) and Mohamed and Kamel, (2019) respectively. The FAdVs types D and E are slightly divergent (Marek et al., 2010), this may raise the need to bivalent or homogenous vaccine to prevent further loses among birds.
Conclusion
The FAdV-2 (species D) is the causative agents of IBH in broiler chickens with 3-5 weeks old age and continue causes periodical outbreaks. Continuous molecular and serological surveillance of FAdVs may provide a worthy database for vaccines and an efficient control strategy development. The variance and divergence among FAdVs types isolated from infected flocks in Egypt should be considered during vaccine selection and manufacturing.
acknowledgements
Authors acknowledge the Department of Avian and Rabbit Medicine at Faulty of Veterinary Medicine-Zagazig University for Technical support.
conflict of interest
The authors declare no conflict of interest.
authors contribution
Yara F. El-Basrey: Sampling, Data curation, analysis, Methodology, writing, Funding acquisition and Resources. Rehab. I Hamed: Sampling, Methodology, writing and Data analysis. Mahmoud Mohamed: Conceptualization, Data curation, Formal analysis, Software, Visualization, Writing - review & editing. Mohamed A Lebdah: Conceptualization, Methodology, Investigation, Resources, Supervision, Validation, Writing - review & editing.
References