Journal of Animal Health and Production
Research Article
Prebiotic Roles of Ocimum gratissimum Extract in the Control of Colibacillosis in Broilers
Onyeka Michael Ikele1*, Ifeoma Maureen Ezeonu2, Chibuzo Nneka Umeh1
1Nnamdi Azikiwe University, Awka, Nigeria; 2University of Nigeria, Nsukka, Nigeria.
Abstract | Prebiotics are non-digestible substances that provide beneficial physiological effect on the host when consumed, by selectively stimulating the favourable growth or activity of beneficial bacteria such as Lactobacilli. Ocimum gratissimum is a Nigerian leafy vegetable known for its vast bioactive compounds and therapeutic use. This research aimed at identifying the prebiotic compounds present in O. gratissimum ethanolic extract and to observe their roles in the control of colibacillosis in broilers; as a natural alternative to antibiotic therapy. Prebiotics present in O. gratissimum were assessed using ethanolic extraction and thin layer chromatography. A 1.5 ml aliquot of 106 cfu/ml E. coli O157:H7 isolated from Nono (sour milk) and identified with 16s rDNA sequencing, was used to elicit colibacillosis infection in three weeks old broilers. Ethanolic extract of O. gratissimum (40 g/L) was orally administered, Norfloxacin (15 g/L) was used as the antibiotic control, uninfected broilers were used as healthy control and broilers infected without treatment were used as negative control for the experiment which was monitored over a period of four weeks. Data were analyzed using Tukey test for mean partitioning. Prebiotics present in O. gratissimum were mainly fructooligosaccharides. Administration of the plant prebiotics to the infected birds resulted in appreciable weight gain (P =0.015), decrease in total E. coli count (P =0.006) and increase in total lactic acid bacteria count (P = 0.025) in the caecum. Histopathology examination of the caecal tissues revealed mucosal lipidosis in the caecum, which indicates probably short chain fatty acid production from prebiotic fermentation by beneficial bacteria that inhabit the colon. O. gratissimum prebiotic administration can serve as an antibiotic alternative to control the colibacillosis in broilers, which poultry farmers can be encouraged to adopt to limit the cases of microbial resistances from poultry farms.
Keywords | Prebiotics, Ocimum gratissimum, E. coli, Broilers, Thin layer chromatography
Editor | Asghar Ali Kamboh, Sindh Agriculture University, Tandojam, Pakistan.
Received | May 16, 2020; Accepted | September 3, 2020; Published | October 10, 2020
*Correspondence | Onyeka Michael Ikele, Nnamdi Azikiwe University, Awka, Nigeria; Email: mo.ikele@unizik.edu.ng
Citation | Ikele OM, Ezeonu IM, Umeh CN (2020). Prebiotic roles of Ocimum gratissimum extract in the control of colibacillosis in broilers. J. Anim. Health Prod. 8(4): 206-211.
DOI | http://dx.doi.org/10.17582/journal.jahp/2020/8.4.206.211
ISSN (Online) | 2308-2801
Copyright © 2020 Ikele et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Antibiotic resistance has been a global threat to disease treatment and this extends from humans to food animals. Alternatives to antibiotic treatments are being investigated in order to control antibiotic resistance rate in bacteria and one of such alternatives is the use of prebiotics. Prebiotics are non-digestible substances that provide beneficial physiological effect on the host when consumed, by selectively stimulating the favourable growth or activity of beneficial bacteria such as Lactobacilli and Bifidobacteria (Roberfroid, 2007; Seifan et al., 2019; Tomasik and Tomasik, 2020). Commonly known prebiotics are oligofructose, inulin, galactooligosaccharides, lactulose and Breast milk oligosaccharides (Seifan et al., 2019; Anandharaj et al., 2020; Durazzo et al., 2020). Prebiotics have been identified from fruits and vegetables such as Chicory, bananas, Vernonia amygdalina (Onugbu) and Ocimum gratissimum (Nchuanwu) (Ezeonu et al., 2016; Durazzo et al., 2020). The prebiotic activities of V. amygdalina and O. gratissimum aqueous leaf extracts in the protection of rabbits (Oryctolagus cuniculus) have been demonstrated by Ezeonu et al. (2012). O. gratissimum is one of the most common leafy vegetables consumed by Nigerians. It appears usually as a small shrub with many branches and simple oval leaves. The leaves are used as food additives, where it serves medicinal and nutritive values, as well as add aroma or flavor to the food (Okoye and Madumelu, 2013). According to Edeoga and Eriata (2011), it is mainly used as spice, and also serves a lot of medicinal purposes which include antibacterial, antifungal, and antihelminthic, beyond its medicinal uses is also a prebiotic potential.
Escherichia coli has been reported as one of the top seven pathogens of public health concern (CDC, 2014). Avian pathogenic E. coli (APEC) is the pathogen responsible for chicken colibacillosis (Lutful-Kabir, 2010). Colibacillosis is an intestinal infection that leads to mortality in broilers from one day to five weeks old (Lutful-Kabir, 2010). The most common method used by Nigerian farmers for controlling this infection is the use of antibiotics, and it has a limitation of increased rate in drug resistance by bacteria because of increased antibiotic float from farm-to-fork. Hence, the adoption of prebiotic administration as a natural means of bacterial infection control is being suggested by many workers (Ezeonu et al., 2016; Seifan et al., 2019; Anandharaj et al., 2020; Durazzo et al., 2020; Slizewska and Chlebicz-Wojcik, 2020; Tomasik and Tomasik, 2020). The objectives of this research were to identify the prebiotics present in O. gratissimum extract using thin layer chromatography; assess the prebiotic roles of the extract on body weights, caecum E. coli and lactic acid bacteria burden; and on the caecum histopathology of broilers infected with E. coli (colibacillosis).
MATERIALS AND METHODS
Isolation of organism
Escherichia coli isolates were obtained from Nono using one in ten fold serial dilutions in sterile peptone water (Titan Biotech, India), and culturing on Eosin methylene blue agar (Sigma-Aldrich Chemie GmbH, India). Cultures were incubated at 35°C for 24 h aerobically according to the methods of Makut et al. (2014).
Identification of organism
Escherichia coli isolates was presumptively identified using routine biochemical tests. Confirmation of isolates was by 16s rDNA molecular typing, carried out at Macrogen Incorporate, South Korea.
Screening of E. coli isolates for avian pathogenicity
Twenty, three-week old broiler chicks were orally infected with 107 cfu/ml of E. coli in phosphate buffered saline, with the aid of a sterile Pasteur pipette. The chicks were then monitored for thirty days for pathological signs such as malaise and occurrence of watery and bloody stools (Ezema, 2013).
Prebiotic assessment of O. gratissimum
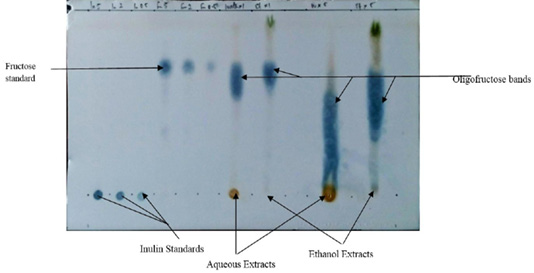
Prebiotic assessment was performed according to the methods described by St. John et al. (1996) and Ezeonu et al. (2016). Ten grams each of room-dried and grounded leaf samples were immersed in 50 ml distilled water and 50 ml of 85% ethanol in a beaker for 24 h. The extracts were obtained by sieving the soaked leaves with Whatmann No. 1 filter paper and allowed for 72 h for evaporation of the solvents and concentration of the extracts. A distance of 0.5 cm was measured from the bottom of thin layer chromatography silica gel 60 F (Merck) plate. Using a pencil, a line was drawn across the plate at the 0.5 cm mark. Aqueous and ethanolic extracts of O. gratissimum were spotted on the plate on separate lanes, alongside with Inulin and Fructose standards, equally on separate lanes. The thin layer chromatograhpy plate was developed in Butanol-Actone-Water (5:4:1 v/v/v). The fructo-polysaccharides present in the plant extracts were visualized by spraying the chromatogram with Urea–Phosphoric acid reagent. The sprayed and air dried chromatogram was placed in an oven set at 110oC for 5-10 min.
Chromatogram was scanned with HP 4890 digital scanner and densitometry of scanned images were quantitatively analyzed using UN-SCANT-ITTM gel software, version 6.1 (Silk scientific, Orem, Utah).
Standardization of pure culture
A 0.25 ml aliquot of pure culture of Avian pathogenic E. coli (APEC) was grown in 25 ml Nutrient broth (Sigma-Aldrich Chemie GmbH, India) and incubated aerobically without agitation at 30°C for 24 h. The cultures were serially diluted using 1 in 10 fold dilution to achieve concentrations of 107 cfu/ml according to the method described by Hartmann et al. (2011).
Animals
Broiler chicks (Gallus domesticus) were obtained from Aroma Farms, Awka, Anambra State Nigeria, as day-old chicks and were raised in battery cages with wood saw dust as bedding materials, at temperature of 30-33°C till they got to three weeks old before they were used for a four-week experiment. They were vaccinated for New castle disease, provided with feeds starting with top starters for the first seven days, and top finishers for the remaining weeks (Table 1). No other antibiotic was administered to the chicks. The overall care of the birds were maintained in accordance with the provisions of the National Institute of Health Guidelines for Care and Use of Laboratory Animals (PUB No. 85-23, revised 1985) and under approval of University Ethical Committee on the use of laboratory animals.
Experimental procedure
Four groups of three-weeks old chicks (n = 10 in each group) were set up as follows: Group A, Healthy control; Group B, Infected with APEC without treatment; Group C, Infected with APEC and treated with 15 g/L Norfloxacin (Norflox-200, Interchemie, Netherlands) as antibiotic control; Group D, Infected with APEC and treated with 40 g/L Ocimum gratissimum extract.
Table 1: Proximate composition of feed used for growth of broilers.
| Nutrient (g/100g) | Starter | Finisher |
| Protein | 18.00 | 10.68 |
| Fat | 11.13 | 11.52 |
| Moisture | 27.96 | 12.62 |
| Ash | 8.27 | 12.25 |
| Carbohydrate | 34.64 | 52.93 |
| Metabolizable energy (Kcal/Kg) | 2106.00 | 2543.20 |
Groups B, C, and D were orally dosed initially with 1.5 ml of 1.3 x 107 cfu/ml of Escherichia coli mixed with 0.5 ml phosphate buffer saline (pH 6) with the aid of a sterile pipette and left for a period of two days to give room for proper pathogen incubation and disease establishment. Afterwards, Group C and D were administered with 15 g/L Norfloxacin and 40 g/L of O. gratissimum ethanolic extract respectively in order to control the infection; while group B was left without treatment (Pascual et al., 2009).
Effect of oral administration of O. gratissimum on growth performance of chicks
Weights, percentage weight gain and specific growth rates of the chicks were monitored. The body weights of the chicken were appropriately taken weekly using Metler weighing balance of 0.01 g sensitivity.
Effect of oral administration of O. gratissimum on caecal microflora of chicks
Caecal lavage was performed on dead chickens from each group with 1 ml of phosphate buffer saline; lavage fluid was diluted using 1 in 10 fold serial dilution and plated on Eosine Methylene Blue (EMB) agar (Sigma-Aldrich Chemie GmbH, India) with aerobic incubation and De Mann-Rogosa and Sharpe agar (Titan Biotech, Rajasthan, India) with anaerobic incubation at 35-37°C. Colony forming units from lavage cultures after 24 h were used to determine caecal E. coli and Lactic acid bacteria burden respectively (Pascual et al., 2009; Ikele et al., 2019).
Histopathological examination of caecal tissues
Caecum samples from each chicken group were excised and immediately fixed in 10% neutral buffered formalin. The tissues were dehydrated into graded concentrations of alcohol (70%, 80%, 90% and 100%) for one hour each. The tissues were cleared overnight using 100 ml of xylene. After blocking using soft paraffin, serial sections of 4µm thickness were made and stained with haematoxylin and eosin stain and photomicrograph was immediately taken (Ikele et al., 2014).
Statistical analyses
One-way ANOVA was applied to determine the means at 95% confidence interval. Tukey test was used for the comparison of means. Data were expressed as mean±SD. The significance threshold was at P≤0.05. All the experiments were biological triplicates.
RESULTS and Discussion
Identification and characterization of isolate
The E. coli isolate that had the most pathogenic effects on test chicks was identified through molecular typing as E. coli O157:H7 strain Sakaii.
Prebiotic assessment of O. gratissimum
Thin layer chromatography assessment of the prebiotics present in O. gratissimum showed the presence of fructooligosaccharides as shown in Figure 1. The prebiotic present in water extract fraction was 24.904 while that of ethanol fraction was 14.210 as shown in Table 2.
Table 2: Thin layer chromatography showing the prebiotic constituents of Ocimum gratissimum.
| Solvents | Prebiotics present (mg/g sample) | ||
| Inulin | Fructose | Fructooligosaccharides | |
| Water extract | 0 | 9.270 | 24.904 |
| Ethanol extract | 0 | 12.311 | 14.210 |
Effects of O. gratissimum administration on mean weights of test broilers
O. gratissimum prebiotic administration gave a significant (P = 0.015) increase in mean weights of test chicks (Figure 2) over a 4-week monitoring period.

Figure 2: Mean body weights of the experimental broilers (4 weeks). A: Healthy control; B: Infected with E. coli but without Treatment; C: Infected with E. coli and treated with Norfloxacin (15g/L); D: Infected with E. coli and treated with Ocimum gratissimum (40g/L).
Effects of O. gratissimum Administration on Caecal Microfolra of Test Broilers
There were reduced E. coli counts (P = 0.006) and increased lactic acid bacteria counts (P = 0.025) in the caecum of the broiler chicks with the periodic administration of O. gratissimum prebiotics as shown in Figures 3 and 4.
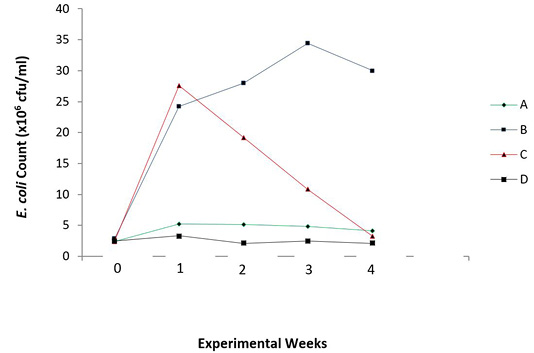
Figure 3: Total E. coli count in the caecum of experimental broilers. A: Healthy control; B: Infected with E. coli but without treatment; C: Infected with E. coli and treated with Norfloxacin (15g/L); D: Infected with E. coli and treated with Ocimum gratissimum (40g/L).
Effects of O. gratissimum administration on caecal tissues architecture of test broilers
Figures 5 and 6 show the histopathological examination of the caecal tissues of the broilers. Prebiotic administration elicited vacoulations which are on-set of mucosal lipidosis and production of short chain fatty acids, which aid in repair of damaged tissue walls through immune-modulation (Plate 3).
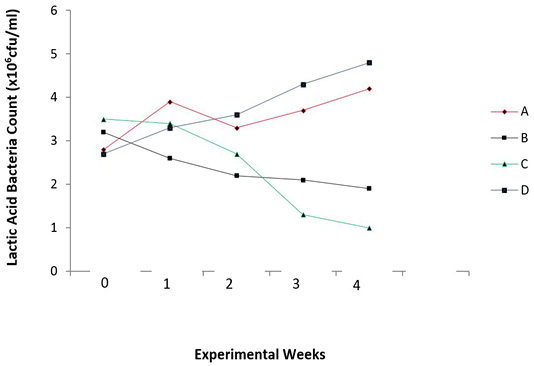
Figure 4: Total lactic acid bacteria count in the caecum of experimental broilers. A: Healthy control; B: Infected with E. coli but without treatment; C: Infected with E. coli and treated with Norfloxacin (15g/L); D: Infected with E. coli and treated with Ocimum gratissimum (40g/L).
There was absence of Inulin in the examined ethanol extracted leaves while there was presence of fructooligosaccharides as prebiotics (Table 2 and Figure 1). Water and Ethanol were used as solvents for prebiotic extraction since both solvents are proctic in nature. It is seen that aqueous extract yielded more fructooligosaccharides than the ethanolic extract, however, ethanolic extract was used for the study as a novel approach to in vivo prebiotic study in broiler chicks. The presence of fructooligosaccharides in the plant extracts agrees with the findings of Ezeonu et al. (2012). The report of Ezeonu et al. (2012) suggested that green leafy vegetables such as Vernonia amygdalina and O. gratissimum could contain different classes of prebiotics.
There was steady increase in weight of healthy uninfected chicks (Group A) compared with other groups. The infected, but untreated chicks (Group B) decreased in weight from about two weeks till the end of the experiment, while the infected birds treated with antibiotics and O. gratissimum (Group C and Group D) showed increased weights by the end of the monitoring period (Figure 2).
There was also a steady increase in E. coli count of infected and untreated chicks (Group B), from the first week to the third week of monitoring, compared with other groups. However, there was a slight decrease in the count on the fourth week, which is still also highest in value when compared to other groups. The antibiotic treated chicks (Group C) showed a sharp decline in E. coli count after the first week of infection, The antibiotic treated chicks (Group C) showed a sharp decline in E. coli count after the first week of infection. The uninfected chicks (Group A) and O. gratissimum treated group (Group D) showed similar patterns in E. coli counts in the caecum.
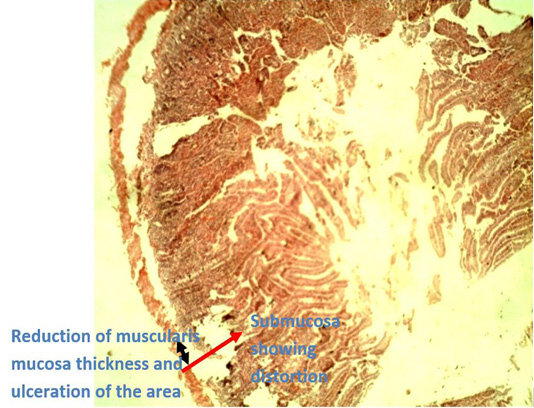
Figure 5: Photomicrograph of caecum of Group B (infected with E. coli but without treatment) showing reduction of thickness of the muscularis mucosae (black arrow head) due to ulceration of the area which went deep into the submucosa (red arrow) leading to ulcerative.

Figure 6: Photomicrograph of caecum of Group D chicken (infected with E. coli and treated with O. gratissimum extract) showing: (1) Minor hemorrhage in the lumen (oval shape) (2) Distorted muscularis mucosa (double arrow head). (3) Vacuolation present keeping for minor onset of accumulation of lipids which is one of the roles played by prebiotics. (H and E. mag. 100X). The prebiotic was able to maintain the caecal architecture of the infected chicks.
Steady increase in Lactic acid bacteria (LAB) count was noticed in the caecum of O. gratissimum prebiotic controlled group (Group D). The antibiotic treated chicks (Group C) showed a decline in lactic acid bacteria count due to its prolonged use in the control of colibacillosis in the chicks. The pathogen was being controlled, however the beneficial bacteria were also affected. The uninfected chicks (Group A) and O. gratissimum treated group (Group D) showed similar patterns in lactic acid bacteria (LAB) counts in the caecum with Group D having higher values in cfu/ml of LAB; and this represents the capacity of the plant extract to stimulate the selective growth of beneficial bacteria, thus substantiating its prebiotic capacity (Figure 4). This finding agrees with the explanations of Ezeonu et al. (2012) on O. gratissimum prebiotics. Slizewska and Chlebicz-Wojcik (2020) also reported the stimulation of different lactic acid bacteria strains with the administration of prebiotics from plant silage to monogastric animals.
Histopathological examination of the caecal tissues of negative control group (Group B) and prebiotic controlled group (Group D) show appearances of ulceration of the caecum by E. coli infection and points of vacoulation for mucosal lipidosis to occur in the caecum, respectively (Figures 5 and 6). The presence of vacoulations is an innate immune response elicited by prebiotics as a means to stimulate accumulation of lipids (mucosal lipidosis) produced during their fermentation by beneficial organisms that inhabit the colon. These produced short-chain fatty acids are used to repair degenerated areas of the caecum tissues (Hosono et al., 2003; Seifan et al., 2019; Anandharaj et al., 2020).
Conclusions and Recommendations
This research has shown that Ocimum gratissimum prebiotics can play vital roles in the control of colibacillosis in broiler chicks and ethanol is also an effective solvent for the extraction of its prebiotics and can serve as an alternative to the use of aqueous extraction which is the usual practice.
Author’s Contribution
Ikele MO conceptualized and carried out the research work, manuscript writing and data analyses. Ezeonu IM assisted in the design adjustment of the research, second supervision and manuscript proof-reading. Umeh CN first designed and supervised the research work before Ezeonu IM completed the supervision.
Conflict of Interest
The authors have declared no conflict of interest.
References