Journal of Animal Health and Production
Research Article
Molecular Detection and Risk Factors of African Horse Sickness Virus (AHSV) in Different Governorates of Sudan
Albagir GM Ahmed1*, Eman O Bakri2, Mohammed O Hussien2, Mahdi EE Taseen3, Abuobaida M Ahmed4, Mohammed A Abdalla5
1Directorate of Livestock Risk assessment, Ministry of Environment, Water and Agriculture, Riyadh, Kingdom of Saudi Arabia; 2Central Laboratory, Ministry of Higher Education and Scientific Research, Khartoum, Sudan; 3Directorate of Livestock, Ministry of Animal Resources Rehed al Birdi locality, Southern Darfur state, Sudan; 4College of Veterinary Medicine, Sudan University of Science and Technology, Khartoum, Sudan; 5Directorate of Animal health, Ministry of Animal Resources and Fishers, Khartoum state, Khartoum, Sudan.
Abstract | African horse sickness (AHS) is one of the most prevalent vector born viral diseases that threaten the equine industry in Sudan. A study was carried out during fall 2018 and 2019 to estimate the prevalence of the disease in horses, to detect the virus in Culicoides spp. (Diptera: Ceratopogonidae) pools and assess the contribution of major risk factors for the occurrence of the disease in horses. Whole blood samples (n=184) were collected randomly from horses from two governorates in Khartoum state and three provinces in Southern Darfur state. In addition, 1916 insects in 18 pools, the pool range 70-150 non-engorged females of different species of Culicoides biting midges were collected from Khartoum governorate and tested for the presence of ribonucleic acid of African horse sickness virus (AHSV) using one-step reverse transcriptase polymerase chain reaction technique. The obtained results revealed that the overall prevalence of AHSV in horses was 38.5% and from the 18 pools of Culicoides spp. the virus was detected in 6 pools (33.3%). Furthermore, in the univariate analysis, risk factors such as locality (p = 0.0001), age (p = 0.018) and sex (p = 0.011) were significantly associated with the prevalence of AHSV in horses. However, the breed of horses did not show statistically significant associations (p>0.05). In the multivariate analysis, locality (OR = 28.271, p = 0.0002) was found to be the most statistically significant risk factor for the occurrence of AHSV. The results of this study highlight that the overall prevalence of AHSV was high in the areas surveyed. The high abundance of the Culicoides recognized as potential AHSV vectors, suggests possible risk of the emergence of AHSV as pandemic in the study area.
Keywords | Horses, African Horse Sickness Virus, Culicoides, RT-PCR, Epidemiology.
Received | June 29, 2020; Accepted | July 27, 2020; Published | October 10, 2020
*Correspondence | Albagir GM Ahmed, Directorate of Livestock Risk assessment, Ministry of Environment, Water and Agriculture, Riyadh, Kingdom of Saudi Arabia; Email: amgbagir@gmail.com
Citation | Ahmed AGM, Bakri EO, Hussien MO, Taseen MEE, Ahmed AM, Abdalla MA (2020). Molecular detection and risk factors of african horse sickness virus (ahsv) in different governorates of sudan. J. Anim. Health Prod. 8(4): 199-205.
DOI | http://dx.doi.org/10.17582/journal.jahp/2020/8.4.199.205
ISSN | 2308-2801
Copyright © 2020 Ahmed et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
African horse sickness (AHS) is a fatal vector-borne viral disease of horses, and generally sub-clinical disease in other Equidae such as mules and donkeys, while zebra is considered a reservoir for the disease and some studies pointed to its importance in continuing the emergence of the disease in Africa (Radostits et al., 2007). Dogs may be infected due to consuming horsemeat from horses that have died from the disease (Van Rensburg et al., 1981). However, recent reports in South Africa showed that dogs got the infection by exposure to Culicoides attack (Van Sittert et al., 2013; Nicolize et al., 2018). Mainly the clinical signs and lesions of the disease result from selective increase of vascular permeability characterized by an impairment of the respiratory and circulatory systems (Radostits et al., 2007). The disease was previously classified by the World Organization for Animal Health (OIE) as a list A disease because of its high morbidity and mortality rates but currently considered as a ‘notifiable’ disease (OIE 2019). It is endemic in most sub-Saharan African countries, however occasional outbreaks have occurred in northern Africa (1965, 1989–1990, 2007–2010), the Middle East (1959–1961), and in Europe (Spain: 1966, 1987–1990, and Portugal: 1989) (Sanchez-Vizcaíno, 2004).
African horse sickness virus (AHSV) containing nine serotypes belongs to the genus Orbivirus of the Reoviridae family. The virus is similar in morphology and shares many biochemical properties with orbiviruses, such as equine encephalosis virus, bluetongue virus and epizootic haemorrhagic disease virus (Coetzer and Guthrie, 2004). It is considered a group of double-stranded RNA viruses (dsRNA) with ten parts of the genome encoding seven structural proteins (VP1-VP7) and four non-structural proteins (NS1, NS2, NS3, NS3A) (Wilson et al., 2009; Fowler et al., 2016). The transmission of the virus is biologically exclusively by Culicoides spp. (Diptera: Ceratopogonidae), of which Culicoides imicola represents a major role in the transmission of the disease. Female insects need an adequate dose of animal blood to be able to lay out eggs, causing the spread of the disease among the vulnerable horses (Borkent 2005; Meiswinkel et al., 2004). There have been many studies conducted in Southern and West Africa to define the various species of Culicoides spp. and highlighted the most abundant species of it. These studies have shown the extent of biting midges resistance to climate change and clarified its important role in spreading the disease and its re-emergence in sub-Saharan Africa (Fall et al., 2015; Gordon et al., 2015; Nevill et al., 2007). Some reports explained that the virus could be presented in the adult phase of Culicoides spp. throughout the year. This can lead to some outbreaks when the herd immunity declines and the population of equidae becomes under the appropriate risk factors for the disease (Venter et al., 2014). In Sudan, Culicoides imicola were previously collected and viruses of bluetongue disease, epizootic haemorrhagic disease and palyam disease were isolated from them and some other species of Culicoides (Mohammed and Mellor, 1990).
The horse population in Sudan was estimated about 784 thousand heads; most of them are in South Darfur state (MARF, 2009). All the original Sudanese horses are small, lightweight with exception of very few sports horses; they are always working animals. They are used for rides in rural areas, but in urban areas, mainly used for local transportation attached to waggons or carts (Wilson, 2007). A live attenuated vaccine for the AHS disease is locally manufactured in the central laboratory in Sudan and contains four strains (types 1, 3, 6 and 9). Although there is no routine vaccination of horses officially. In 2016, about 124880 doses of the vaccine were produced and the total number of vaccinated horses was 4301 heads (OIE, 2016). Historically, the disease was reported for the first time in eastern Sudan, when the death of horses was as ascribe to African horse sickness in Kassala province, and spreading to Blue Nile province in the same year (Anon, 1903). However, only serotype nine was isolated (Eisa, 1974; Hajer et al., 1980) indicating that serotype 9 might be the prevalent serotype of AHSV in Sudan. There were some serological studies conducted to investigate the disease in horses and donkeys in Sudan (Abu Elzein et al. 1989; Ihsan 2004; Elghazali and Ali, 2013; Karamalla, et al., 2018). Albagir et al. (2018) indicated that the seroprevalence of AHS was significantly associated with the vaccination status of equines, geographic location of states, species, breed, and presence of water bodies.
Recently the RT-PCR procedure has been used as a means of a highly sensitive technique, less expensive and conducted in less time compared to the isolation of the virus. This type of diagnosis supports decision makers positively in applying the rapid response mechanism when the disease breaks out and motivates them to take all control measures that limit the spread of the disease to new areas (Aradaib et al. 2006; Aradaib, 2009). Therefore, the main objectives of this study were to confirm the presence of AHSV in Culicoides as well as in horses, to determine the prevalence of the virus in horses and to identify risk factors that may play a role in spreading the disease in different areas of Sudan.
Materials and Methods
Study Area
This study was conducted in the states of Khartoum and South Darfur. Khartoum state lies between longitudes 31.5 to 34 °E and latitudes 15 to 16 °N. The weather is hot dry in summer and cold dry in winter. The average annual rainfall ranges between 150 mm and 250 mm. The horse population estimated was about 6585 heads (MARF, 2009).
While South Darfur State lies between longitude: 30.8 - 30.13 E and latitude: 23.15 – 28 N. The northern part is located in the semi-desert region, while the southern part is located in the rich woodland savannah region. The weather is hot and dry in summer, while it is cold and dry in winter. In autumn it is warm. The mean of the rainfall is about 362 mm. The estimated horse population in the state is about 750 thousand (MARF, 2009).
Study Design
The study was carried out during the autumn season in September 2018 and completed in November 2019. The sampling sites were selected according to the history of the disease outbreaks by monitoring the epidemiological situation of the disease and following up the monthly reports of the government veterinary clinics in the areas where the disease reoccurred. The study involved a cross-sectional observation in simple random sampling technique (Martin et al., 1987). A total of 184 horses (16 crossbred vaccinated horses and 168 not vaccinated local breed horses) from two governorates in Khartoum State and three localities in Southern Darfur State were randomly sampled and investigated. In addition, 1916 insects in 18 pools, the pool range 70- 150 non engorged females of different species of Culicoides biting midges were tested for the presence of AHSV RNA using one-step reverse transcriptase polymerase chain reaction (RT-PCR) technique. Samples were collected from private stables during the autumn season of the year 2018 in Khartoum North and Khartoum governorates in Khartoum state. In South Darfur state, the samples were collected at the end of the Autumn of the year 2019 from livestock markets in Nyala, Edd al Fursan and Rehed al Birdi. The samples were collected from horses offered for sale from neighboring villages in the governorate’s livestock market. All horses were in good health condition and did not show symptoms of the disease. All data of the owner of the animals and the source of these animals were recorded, in addition to animal-related information such as sex, age and vaccination against the disease.
Collection of Blood samples
Whole blood samples were collected from horses in the early morning. Five ml samples of whole blood were transported in a sterile manner using a method that considers animal welfare by puncturing the jugular vein to the sterile tubes with ethylene-diamine-tetra-acetic (EDTA), which were stored at 4 ° C and then stored at -20 ° C until examined in the laboratory (Barrelet and Ricketts, 2002).
Culicoides Collection
Culicoides spp. were collected during daylight hours by using a locally made Aerial net as (Michael, 2001), in the morning half hour before sunrise and continuing for an hour after sunrise as well in the evening last hour before sunset and continue collecting after sun set until dark. In addition, Culicoides were collected using a light trap (Homemade as explained by Venter et al., 2009) close to the horse stables. The biting midges were sorted from the rest genera of family Ceratopogonidae based on the wings, which have contrasting dark and milky white spots, using the taxonomic key of Glukhova (1989) under an SZX 16 stereoscopic microscope (Olympus) and counted. Collected Culicoides were preserved in 70% ethanol until tested in the laboratory.
Viral Nucleic Acid Extraction
The innuPREP Virus RNA extraction kit (analytic Jena, Jena, Germany) was used to extract viral nucleic acids from the whole blood, pools of Culicoides. RNA was extracted according to the manufacturer’s instructions, and the final concentration of RNA was stored at -80 °C.
Reverse Transcriptase Polymerase Chain Reaction (RT-PCR)
To conduct the polymerization process, a commercially live-attenuated vaccine of AHSV was used to prepare the positive control after extracting the antigen from the vaccine (two vials of African horse sickness Vaccine: Reg. No. G 0116, consists of six serotypes 1, 2, 3, 4, 6, 7), imported from Onderstepoort, South Africa. The reaction was done using a set of primers given as follows: (Foreword: 5′-GCGCCACCAATTGGAGATGT-3′) and (Reverse: 5′- TCCCTCTCCTCCTCTGTGT-3′) (Hussien et al., 2019). The reaction was completed according to instructions of kit manufacturer (Analytik Jena, Germany) and an ending volume of 20µl was used in one step RT-PCR, containing 10 µl 2x RT-PCR buffer, 1 µl Reverse transcriptase (RT) enzyme, 2 µl primer mix in a concentration of a 5 panel, 5 µl RNA template and 2 µl nuclease – free H2O. Amplifications were performed for 15 min at 45°C and for 10 min at 95oC for RT enzyme initial activation followed by 40 cycles (94°C for 30 Sec, 57°C for 30 Sec, and 72°C for 45 Sec) and final extension for 10 min at 72°C (K960 gradient thermal cycler, Heal Force, China). The PCR product was exposed to electrophoresis using a 1.5% agarose gel for 30 min at 120 V and then imagined under UV light after staining with ethidium bromide. The predictable size of band is 221 bp.
Statistical Analysis
The data were entered into Microsoft excel spreadsheet. The SPSS version 21 and Arc GIS 10.3, software were used for the analysis of data. Data were investigated descriptively in the first step, using the frequency table and cross tabulation. Then the relationship of the potential risk factors with AHSV at the individual level was examined using the Chi-square test. The level of significance was set at P<0.05. For the investigation of the association between the prevalence in response to individual animal characteristics, a multivariate analysis was carried out using the logistic regression. The strength of association between the prevalence and the risk factors of AHSV was measured using the OR (odds ratio) and the level of significance was set at p ≤ 0.05.
Results
Prevalence of AHSV
The analytical sensitivity of the PCR used in this study was ten-fold dilutions made from AHSV vaccine strain (3.7 TCID50/ml) commonly used in Sudan, and used as templates for the PCR reaction. The detection limit of the PCR was 104. AHSV RNA was successfully detected in whole blood samples and Culicoides pools in the study areas (Figure 1). The overall prevalence of AHSV RNA in horses and from the 18 pools of Culicoides the virus was detected in 6 pools (33.3%). The highest prevalence of the AHSV RNA in horses was reported in Edd al Fursan locality (86.4%), while Khartoum North governorate in Khartoum State showed the lowest prevalence of the virus (14.8%) (Figure 2). The prevalence of the virus was high in female horses (51.7%) and within age groups; the prevalence was high in old horses (47.7%). Regard to the breed of horses the prevalence of the virus was high in crossbred horses (50%). (Table 1). There was a coincidence between the presence of the virus in insects and the high prevalence of the disease (82.3%) in horses was observed only in Khartoum governorate. Where there was 14/17 (82.3%) of horses were infected in the stable, which close to the location where AHSV RNA was detected in Culicoides spp.
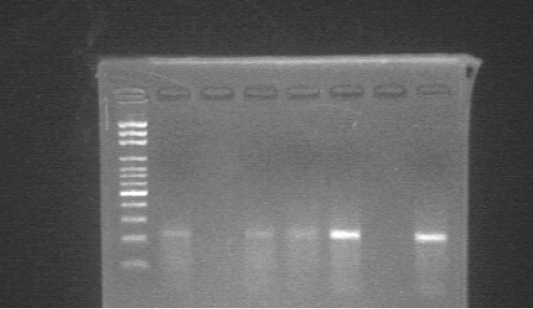
Figure 1: Agarose gel electrophoresis of the products amplified by RT-PCR using specific primers for AHSV. M; 100 bp DNA ladder, Lane 1,3,4,5; positive samples, Lane 2; negative sample, Lane 6; negative control, Lane 7; positive control.

Figure 2: The map of Sudan showing the prevalence of African horse sickness virus in different governorates and localities of Sudan and the detection of the virus in Culicoides biting midges in Khartoum govern.
Table 1: Univariate analysis of the association of potential risk factors for AHSV in horses in some localities in Sudan*.
| Variables | No. of samples tested | +ve (%) |
2 |
P- value |
|
Locality: Khartoum North Khartoum Nyala Rehed al Birdi Edd al Fursan Total |
27 36 49 50 22 184 |
4 (14.8%) 15 (21.7%) 14 (28.6%) 19 (38.0%) 19 (86.4%) 71 (38.5%) |
29.855 | 0.000 |
|
Breed: Local Cross breed |
168 16 |
63 (37.5%) 8 (50 %) |
0.963 | 0.326 |
|
Sex: Male Female |
124 60 |
40 (32.3%) 31 (51.7%) |
6.428 | 0.011 |
|
Age: Young (1-4 years) Old (above 4 years) |
98 86 |
30 (30.6%) 41 (47.7%) |
5.627 | 0.018 |
*AHSV: African horse sickness virus
Associated Risk Factors of AHSV
The association between the occurrence of AHSV in the selected horses and different potential risk factors was shown in Table 1, risk factors such as locality (p = 0.0001), age (p = 0.018) and sex (p = 0.011) were significantly associated with the prevalence of the virus in horses. However, the breed of horses did not show statistically significant association (p>0.05). As shown in Table 2, the odds ratio was used to quantify the strength of association between potential risk factors and the prevalence of AHSV. Horses in Edd al Fursan was almost 28 times more likely to be infected compared with horses in Khartoum North governorate (OR = 28.271, p = 0.0002). Also, horses in Khartoum governorate were almost 3 times more likely to be infected compared with horses in Khartoum North governorate (OR = 3.425, p = 0.058).
Discussion
There have been some molecular studies in Khartoum state in Sudan developed to detect the virus in cell culture (Aradaib et al. 2006; Aradaib, 2009). However, the present study is considered to be the first to estimate the prevalence of the virus and investigate the contribution of major risk factors for the occurrence of the disease in horses in several governorates in Sudan, where the disease has appeared recently. The current study indicated an overall prevalence of AHSV in horses as 38.5% and from the 18 pools of Culicoides spp. the virus was detected in 6 pools (33.3%). Despite the early appearance of the disease in
Table 2: Multivariate analysis of potential risk factors associated with the AHSV in horses in some localities in Sudan.
| Risk Factors | No. of samples tested | +ve (%) | OR | 95% Cl for OR | P-value |
|
Locality: Khartoum North Khartoum Nyala Rehed al Birdi Edd al Fursan |
27 36 49 50 22 |
4 (14.8%) 15 (21.7%) 14 (28.6%) 19 (38.0%) 19 (86.4%) |
Ref. 3.425 1.738 2.703 28.271 |
0.960 – 12.219 0.484 – 6.235 0.776 – 9.408 5.491 – 145.560 |
0.058 0.397 0.118 0.0002 |
|
Sex: Male Female |
124 60 |
40 (32.3%) 31 (51.7%) |
Ref. 1.889 |
0.929 – 3.845 |
0.079 |
AHSV: African horse sickness virus; CI: Confidence interval; OR: Odd ratio
eastern Sudan and its transmission to the Blue Nile Governorate, it has recently become endemic in most areas in the states of Khartoum and South Darfur. The prevalence results were high in the localities of Edd al Fursan, Rehed al Birdi and Nyala in Southern Darfur state 86.4%, 38% and 28.6% respectively. In this state, most horses belonged to nomads and weren’t available for veterinary treatment during the critical rainy season. The disease in this state undoubtedly continued to be a problem in native horses. Most owners of affected horses and the authorities were surely aware of this as evidenced by the vaccine offered (national production consist 4 serotypes 1, 3, 6 and 9). The higher prevalence of the disease in this state may refer to all local horses, which sampled in this study, were not vaccinated two years ago. There were statistical significant differences of the prevalence between the various sites in Khartoum state and South Darfur state (p ≤ 0.05) and this result was in close agreement with results reported by Albagir et al. (2018).
The current study further revealed in the univariate analysis (Table 1) that there is a statistically significant variation (p ≤ 0.05) of the prevalence between the local and crossbred horses, a higher prevalence observed in crossbred compared with the local breed. Although the crossbred horses receive high, care by raising them in vector protected stables and vaccinated annually against the disease the prevalence was high. This is probably due to the existence of a problem in the vaccination process or the inefficiency of the vaccine to provide protection against strains not included in it.
Regarding sex, our findings showed significant variation (p ≤ 0 .05) in the prevalence, with a higher prevalence of the virus in females than in males. The high susceptibility of females to the disease was probably due to the availability of several predisposing factors decreasing the immunity during pregnancy and lactation because of some physiological changes (Merlot et al., 2013). However, the risk factor of sex did not remain statistically significant in the final multivariate model. The presence of AHSV in culicoides was found to be greater than those stated in various areas of South Africa (Scheffer et al., 2012; Venter et al., 2014). This variance in our study and other previous reports could probably be due to difference in geographical location, season of sampling, instruments employed and study methods by the researchers. One of the annotations in this study is that the biting midges were trapped by the Aerial net handheld trap were visible, clear and easy to inspect, compared to the ones caught in light trap. Our findings regarding association of sex were in line with the results reported by Scheffer et al. (2012).
Culicoides abundance was relatively high during September to mid-October, and after that it was decreased during late October and early November. Trapping was attempted again in January during the winter season, but the density was very low or not available. Similar observations were described in pervious studies conducted in Sudan and South Africa (Mohammed and Mellor, 1990; Venter et al., 2014). The coincidence between the presence of the virus in Culicoides spp. and the higher prevalence of the disease in horses in Khartoum governorate confirmed the virus circulation in this area. Research is needed to find out which was the first to appear in the infected area, the Culicoides as predisposing factor or the introduction of an infected animal (Martin et al., 1987). Recently, some studies were designed based on the diagnosis of the virus in Culicoides, with the aim of identifying endemic areas. Moreover, this procedure helps towards applying rapid action to limit the transmission of the disease to free areas through the adult Culicoides spp. control program and destruction of habitat for insect reproduction (Grewar et al., 2013; Venter et al., 2014; De Waal et al., 2016).
Conclusion
The present study indicated that the overall prevalence of AHSV was high in the areas surveyed. The disease was more prevalent in Edd al Fursan, Rehed al Birdi and Nyala that requires a review of the vaccine used in these localities. The high abundance of the Culicoides spp., recognized as potential AHSV vectors, suggests possible risk of the emergence of AHSV in other areas of the country. There is a need to identify the different species of Culicoides to know which species are most closely related to disease transmission in equine in Sudan.
Acknowledgements
We thank the Department of animal resource in Nyala and Aid Alfursan localities, Southern Darfur State for permission and their valuable help in blood collection from horses in livestock markets. The high assistance of Dr. Al hadi A. Alteijany and Dr. Adam Fadule is gratefully acknowledged.
Conflict of interest
The authors declare that they have no any material interests that can be construed as a conflict of interest.
authors contribution
Albagir GM Ahmed was responsible for the study design, collection of blood samples and Cullicoides, helped in laboratory work, analyzed the data and wrote the manuscript. Mahdi EE Taseen and Abuobaida M Ahmed helped in collection of blood samples. Eman O Bakri and Mohammed O Hussien performed the laboratory work and helped writing the manuscript. Mohammed A Abdalla supervised the study and revised the manuscript. All authors have participated in discussion of results and approved the final version of this manuscript.
References





