Journal of Animal Health and Production
Research Article
Clinical, Lipid Peroxidation, Antioxidant Status and Hemato-Biochemical Alterations in Tropical Theileriosis Affected Crossbred Cows
Emad Abdel-Hamied1, Shawky Mohamed Aboelhadid2, Waleed Arafa2, Mourad Mahmoud Mahmoud1*
1Department of Animal Medicine, Faculty of Veterinary Medicine, Beni-Suef University, Beni-Suef 62511, Egypt; 2Department of Parasitology, Faculty of Veterinary Medicine, Beni-Suef University, Beni-Suef 62511, Egypt.
Abstract | This study was designed to elucidate the clinical, lipid peroxidation, antioxidant status and hemato-biochemical alterations in tropical theileriosis (TT) affected cows for perfect diagnosis, understanding disease pathogenesis, evaluation of disease severity and prognosis. Based on clinical and laboratory findings, 31crossbred cows were diagnosed as positive for theileriosis, and another11healthy crossbred cows as controls were included in this study. Clinical, microscopic and PCR findings of theileriosis were recorded. PCR results confirmed that Theileria annulata was the causative agent. Blood and serum samples were collected to assess hematological, biochemical and oxidant/antioxidant alterations. The most observed clinical findings were fever, anorexia, lacrimation, oculo-nasal discharge, enlarged superficial lymph nodes, congested mucus membranes, cough and elevated respiratory and heart rates. Hemato-biochemical parameters showed significant decrease (p<0.05) in eryrhrocyte count, hemoglobin content, hematocrit, mean corpuscular hemoglobin concentration (MCHC), total leucocytic count (TLC), neutrophils, serum albumin and iron levels accompanied with significant increase (p<0.05) in lymphocytes, monocytes, serum globulin, bilirubin and AST activity in theileriosis affected cows. A significant increase (p<0.05) in malondialdehyde (MDA) along with relative decrease in total antioxidant capacity (TAC) in the sera of theileriosis affected animals was recorded. In conclusion, tropical theileriosis is associated with significant hemato-biochemical alterations in which anemia and impaired liver function are the main features. Also, the disease induces remarkable oxidative stress with depletion of antioxidant status. Anemia, disturbed liver function and oxidative stress are likely the major determinants of the disease pathogenesis, severity, prognosis and supportive therapy.
Keywords | Theileriosis, Hematology, MDA, TAC, PCR.
Received | March 29, 2020; Accepted | May 27, 2020; Published | September 11, 2020
*Correspondence | Mourad Mahmoud Mahmoud, Department of Animal Medicine, Faculty of Veterinary Medicine, Beni-Suef University, Beni-Suef 62511, Egypt; Email: morad.mahmoud@vet.bsu.edu.eg
Citation | Abdel-Hamied E, Aboelhadid SM, Arafa W, Mahmoud MM (2020). Clinical, lipid peroxidation, antioxidant status and hemato-biochemical alterations in tropical theileriosis affected crossbred cows. J. Anim. Health Prod. 8(3): 150-157.
DOI | http://dx.doi.org/10.17582/journal.jahp/2020/8.3.150.157
ISSN | 2308-2801
Copyright © 2020 Mahmoud et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Tropical theileriosisis (TT) is a dominant hemoprotozoal threatening disease affecting cattle and primarily is caused by the haemoprotozoan Theileria annulata. The disease is one of the main destructive constraints to cattle health and production causing large economic losses due to decreased milk/meat production, high morbidity, mortality and high costs of treatment and control measures (Haque et al., 2010; Kachhawa et al., 2016).The disease may cause up to 80% mortality rate in susceptible cattle (Sandhu et al., 1998). After host infection, the protozoan multiplies in the lymphoid tissues and then invades erythrocytes to complete its life cycle (Soulsby, 1982), causing severe anemia (Jain, 1993). The clinical signs and outcome of TT depend mainly upon the damaging effect of the causative agent on the lymphoid tissues and host susceptibility (Singh et al., 2018). Fever, anorexia, swelling of superficial lymph nodes, nasal and ocular discharges with congestion of the visible mucous membranes, coughing and salivation are the most common clinical findings in acute tropical theileriosis (Osman and Al-Gaabary, 2007). In late stage of the disease, other clinical signs including diarrhea, corneal opacity and subcutaneous edema could be found (Reda, 2012). Moreover, affected cattle usually experience varying degrees of leukopenia and/or anemia (Radostits et al., 2006). Field diagnosis of TT is fundamentally based on the clinical syndrome and tick infestation of the affected animals. Confirmation of such suspected diagnosis depends usually upon demonstration of Koch’s blue bodies inside the lymphocytes and monocytes of either peripheral blood or lymph nodes smears (Aktas et al., 2006). Presence of Koch’s blue bodies inside lymphocytes and monocytes of either lymph node or peripheral blood smears is a pathognomonic finding for the disease (Tayo et al., 2011). Using polymerase chain reaction (PCR) for diagnosis of TT in diseased and carrier state animals has a great advantage of increased both sensitivity and specificity than the traditional techniques (Aktas et al., 2002; Vantansever and Nalbantoglu, 2002). Much of the disease pathology is due to the accompanied alterations in haemato-biochemical variables (Radostits et al., 2006). Moreover, oxidative stress in theileriosis affected animals has been described (Saleh et al., 2011; Singh et al., 2018). Erythrocytes are very prone to oxidative damage because of the abundance of membrane lipids which are the primary targets for oxidation (Davies and Goldberg, 1987). Lipid peroxidation of RBCs membrane resulting in increased membrane permeability, increased RBCs fragility and subsequently anemia (Saluja et al., 1999). Understanding hematological and sero-biochemical changes provide useful information about the disease severity and involvement of certain organ function during the disease course (Modi et al., 2014).Therefore, the present study was designed to describe and understand the clinical, lipid peroxidation, antioxidant and hemato-biochemical alterations associated with TT in naturally infected crossbred cows.
Materials and Methods
Animals and Experimental Design
This study was conducted in Beni-Suef province, Egypt during May to October 2019 where Bovine Tropical Theileriosis (BTT) was prevalent. A total of 42 lactating crossbred cows aged from three to six years were included in the present study and divided into two groups. Diseased group composed of 31 clinically diseased cows showed typical symptoms of theileriosis was selected. Another 11 clinically healthy cows served as control group. All cows were subjected to careful clinical examination. Blood smears from control and diseased cows were prepared and examined microscopically for Theileria spp. detection. Further confirmation was done by molecular detection of Theileria in whole blood samples using PCR. Blood samples were obtained for elucidation of hemato-biochemical variables, antioxidant and lipid peroxidation status in TT affected cows.
Clinical Examination and Sample Collection
All animals under experiment were carefully examined (Radostits et al., 2000) and the clinical data were recorded. Two blood samples were collected from each cow from jugular vein through a clean dry needle into EDTA containing tubes and plain tubes. Hematological analysis was done within the same day of collection and the blood samples were further stored at -20°C for DNA extraction. The second tube without anticoagulant was used to obtain clear non haemolysed blood serum which was carefully taken and immediately stored at -20°C until analysis of the blood biochemical parameters.
Confirmation of T. Annulata Infection in the Examined Animals
Microscopical examination: Three blood films from each animal were prepared, let to dry, fixed by methanol (99.5%) for 1 min and stained for 30 min by diluted commercial Giemsa solution then examined under oil immersion lens of light microscope to confirm T. annulata infection (Zafar et al., 2006). The parasite was demonstrated depending on the characters described by Soulsby (1982).
Molecular diagnosis
DNA extraction: Genomic DNA extraction was done in Faculty of Veterinary Medicine, Beni-Suef, Egypt, using Geneaid, New Taipei, Taiwan DNA extraction kit. DNA samples were stored at −20 °C to carry the PCR amplification.
DNA amplification:Polymerase chain reactions of T. annulata merozoite-piroplasm surface antigen (Tams1) (F 5/-GTT AAT GCT GCA AAT GAG GAT G3/, and R5/-GGACTGATGAGAAGACGATGAG -3) were performed (Kirvar et al., 2000). PCR reaction 25 µL of 12.5 µL 2X master mix, 1 µl of the F primer (10 pmol/µl), 1 µl of the R primer (10 pmol/µl), 3 µl DNA, and 7.5 µl nuclease free water. Cycling conditions were initial denaturation for 5 min at 95 ºC, 37 cycles of denaturation for 30 s at 95 ºC, annealing for 60 s at 54 ºC and elongation for 1 min at 72 ºC. Then the final extension at 72 ºC for 7 min was allowed. Amplified products were visualized on a 1.5% agarose gel under UV transillumination after staining with ethidium bromide.
Hematological Examination
Whole blood samples were used for estimating the hemogram parameters; RBCs, hemoglobin concentration, red blood cell indices, total and differential leucocytic counts were measured according to Feldman et al. (2000).
Biochemical Analyses
Biochemical serum analysis of total proteins, albumin, glucose, urea, creatinine, total bilirubin, direct bilirubin, ALT, AST, Iron and Copper were estimated spectrophotometrically using commercially available chemical kits following manufacturer instructions. The test kits were supplied by Bio-Diagnostic Comp, Cairo, Egypt.
Lipid Peroxidation and Antioxidant Status Evaluation
Lipid peroxidation product (MDA) and total antioxidant capacity (TAC) were estimated using test kits supplied by Bio-Diagnostic Company-Egypt. These parameters were manually determined using spectrophotometer as suggested by manufacturer.
Statistical Analysis
Data were analyzed using SPSS program (SPSS for windows Version 22, SPSS Inc., Chicago, USA). The differences of mean values between both groups were compared using Student’s t-test. Mean values of statistical significance were based on 5% level of significance. Data are expressed as (mean values ± SD).
Results and Discussion
In Egypt, cattle population is prone to serious health problems caused by bovine tropical theileriosis (BTT) that results in great economic losses due to high morbidity, fatality, lowered productivity and increased costs of treatment and preventive measures of the disease. The animals included in the current study were diagnosed as positive or negative for BTT according to clinical, microscopical and PCR findings. Regarding clinical examination, the theileriosis affected group showed typical clinical findings of BTT in which fever (40.60±0.35°Cvs.38.78±0.19°C in theileriosis and control groups respectively), inappetence, depression, lacrimation, nasal discharge, enlarged superficial lymph nodes, increased respiratory and heart rates (39.40±1.14vs.25.60±1.14 cycle/min and 81.60±1.24vs. 57.20±1.64 beat/min in theileriosis and control groups respectively), cough, and congested mucous membranes were the major findings in acute cases. Corneal opacity, loss of condition, sometimes tarry feces, pale to icteric mucous membranes were observed in more prolonged cases. These findings are Similar to the results reported by Radostits et al. (2006), Kohli et al. (2014) and Kachhawa et al (2016). The tissue damage and adverse clinical findings that occur in BTT could be attributed to the uncontrolled proliferation and metastasis of schizont-infected lymphoid cells (Radostits et al., 2006) and the elevated levels of pro-inflammatory cytokines that are produced by parasitized monocytes (Glass et al., 2003). Fever in TT affected cattle may be linked to thermoregulatory center activation due to the release of endogenous pyrogens produced from parasitemia and cell lysis (Glass et al., 2003). Anorexia, depression and congestion of visible mucous membranes might be occurred as consequences to persistent high fever in acute stages of the disease. Enlargement of superficial lymph nodes was attributed to generalized lymphoid proliferation caused by uncontrolled hyperplasia of schizont-infected T-lymphocytes (Radostits et al., 2006). The corneal opacity in advanced cases could be attributed to the leukocytic infiltration (Irvin and Mawmachi, 1983). The observed pallor to icteric mucous membranes could be referred to the pronounced anemia caused by destruction of parasitized RBCs and hyperbilirubinemia in more advanced cases. The observed respiratory signs including nasal discharge, respiratory distress and cough could be due to the developed severe pulmonary edema in advanced cases as a result of released vasoactive substances from disintegrated lymphocytes in lungs (Radostits et al., 2006).
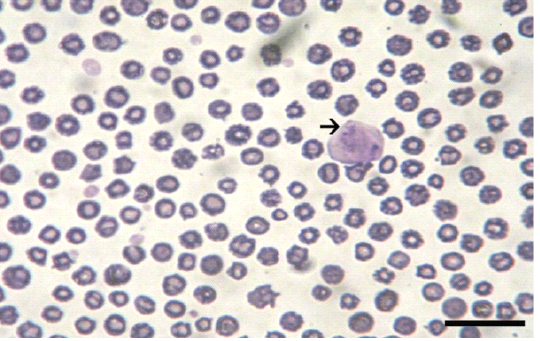
Giemsa stained peripheral blood films from theileriosis affected cows included in the present work revealed presence of the characteristic Koch’s blue bodies inside the lymphocytes (Figure 1) and intra-erythrocytic piroplasms which is pathognomonic for Theileria infection (Tayo et al., 2011). Whereas smears from control cows were free from developmental stages of the protozoan. PCR findings (Figure 2) of blood samples from theileriosis affected group showed the specific amplicon (768 bp) by using specific T. annulata merozoite-piroplasm surface antigen while samples from controls were negative by PCR (Kirvar et al., 2000). These findings proved that T. annulata was the causative agent in the diseased group while the controls were from the pathogen.
Hemogram results are presented in Table (1). Concerning erythrogram, a significant (p<0.05) reduction in RBCs count, Hb content, hematocrit and MCHC% was observed in diseased cases as compared to controls. These findings indicated that cows with tropical theileriosis suffered a pr-
Table 1: Hemogram in healthy control and theileriosis affected cows.
| Parameter | Controls | Theileriosis affected cows |
P-value |
|
RBCs (106/ul) |
7.27±0.75 | 5.84±1.74 | 0.04* |
| HB (g/dl) | 12.16±1.59 | 8.18±0.29 |
˂0.001** |
| PCV (%) | 36.48±4.76 | 24.54±0.88 |
˂0.001** |
| MCV (fl) | 50.93±10.19 | 46.43±19.06 | 0.555 (NS) |
| MCH (pg) | 16.98±3.40 | 15.48±6.35 | 0.56 (NS) |
| MCHC (%) | 33.54±0.59 | 32.31±1.56 | 0.043 * |
|
TLC (103/ul) |
6.59±0.98 | 4.64±2.20 | 0.028* |
| Neutrophils (%) | 36.00±5.42 | 25.00±4.38 |
˂0.001** |
| Lymphocytes (%) | 56.80±5.57 | 66.25±4.65 | 0.001* |
| Eosinophils (%) | 3.30±1.70 | 3.50±1.60 | 0.803 (NS) |
| Monocytes (%) | 3.90±1.10 | 5.25±1.04 |
0.017* |
*Healthy and diseased cows significantly different at p<0.05. **Significant at p<0.001. RBCs= Red blood cells, Hb=Hemoglobin, PCV=Packed cell volume, MCV=Mean corpuscular volume, MCH=Mean corpuscular haemoglobin, MCHC=Mean corpuscular hemoglobin concentration, TLC=Total leukocyte count.
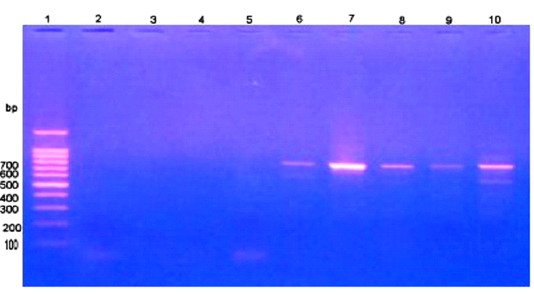
Figure 2: PCR findings of T. annulata infected samples. Ladder of 100 base pair. (2-5) control -ve, (6-10) T. annulata +ve samples of 768 bp.
onounced normocytic hypochromic anemia during the course of the disease. Similar findings were obtained by Khan et al. (2011) and Ganguly et al. (2015). Anemia was a main clinical aspect in diseased cases (Nazifi et al., 2011). Various explanations have been mentioned to describe causes of anemia in tropical theileriosis included the destructive effect of intraerythrocytic piroplasms, erythrophagocytosis of parasitized RBCs by macrophages, suppressed erythropoietic activity (Ugalmugle et al., 2010), damaging effect of toxic metabolites of the protozoan, persistent blood loss caused by blood sucking-ticks (Geerts et al., 2001) and suppressing effects of tumor necrotizing factor- α on erythrogenesis (Boulter and Hall, 2000). Additionally, some published reports suggested a close relationship between anemia and oxidative damage of RBCs (Nazifi et al., 2008).
Shifting to leukogram, there was a significant reduction in TLC and neutrophils with a significant elevation in lymphocytes and monocytes in theileriosis infected cattle (Hussein et al., 2007; Abd El-Hamed et al., 2016). These findings could be due to the large-scale destruction of leukocytes by the protozoan (Abd El-Hamed et al., 2016) coupled with the suppressed leukogenesis caused by the adverse effects of the protozoan toxic metabolites (Hussein et al., 2007). The significant increase in lymphocytes and monocytes in diseased cases might be due to the proliferation of these cells during course of the disease as a host defense mechanism to infection (Modi et al., 2015). The findings in the present work showed that tropical theileriosis induces significant hematological alterations in which anemia is the main aspect.
Biochemical changes are listed in Table (2). Regarding protein picture, the results showed a decrease in the serum total protein (p>0.05) accompanied with highly significant (p<0.001) decrease in the serum albumin in diseased cattle as compared to control (Kachhawa et al., 2016; Devadevi et al., 2018; Abubakar et al., 2019) that could be attributed to the decreased albumin synthesis caused by decreased feed intake and the impaired liver function in diseased cases. T. annulata has harmful effects on hepatocytes of infected animals resulting in hepatic damage in the form of coagulative necrosis, hepatic cords distortion and heavy lymphocytic infiltration in the periportal areas as was explained by Sandhu et al. (1998) who reported macroscopic and microscopic damage in hepatic tissues of calves naturally infected with T. annulata. The liver damage associated with bovine theileriosis could be referred to the harmful effects of the toxic metabolites of the protozoan on the liver (Hussein et al., 2007), inflammatory changes in hepatic cells as a result of trapping of the damaged infected erythrocytes, and lymphocytes (Abubakar et al., 2019) and degenerative changes in liver cells caused by anemic hypoxia.
Serum globulin levels revealed a significant (p<0.05) elevation in diseased group as compared to controls. This could
Table 2: Blood serum biochemical parameters in healthy control and theileriosis affected cows.
| Parameter | Controls | Theileriosis affected cows |
P-value |
| Total protein(g/dl) | 6.91±1.14 | 6.34±1.79 | 0.333 (NS) |
| Albumin (g/dl) | 3.93±0.42 | 3.14±0.27 |
˂0.001** |
| Globulin (g/dl) | 2.28±0.78 | 3.26±0.77 | 0.011* |
| A/G ratio | 1.79±0.39 | 1.02±0.30 |
˂0.001** |
| Glucose (mg/dl) | 66.72±12.92 | 69.76±9.62 | 0.559 (NS) |
| Urea (mg/dl) | 14.56±4.56 | 19.30±5.93 | 0.06 (NS) |
| Creatinine (mg/dl) | 2.27±0.30 | 2.34±0.46 | 0.688 (NS) |
| Total bilirubin(mg/dl) | 0.39±0.29 | 2.20±1.94 | 0.009* |
| Direct bilirubin(mg/dl) | 0.32±0.16 | 0.76±0.42 | 0.006* |
| Indirect bilirubin(mg/dl) | 0.07±0.02 | 1.43±0.120 |
˂0.001** |
| ALT (U/L) | 14.31±2.19 | 15.42±4.00 | 0.576 (NS) |
| AST (U/L) | 24.64±3.37 | 34.86±10.77 | 0.042* |
| Iron (ug/dl) | 142.56±13.56 | 64.39±4.77 |
˂0.001** |
| Copper (ug/dl) | 110.81±12.52 | 109.06±17.34 |
0.861 (NS) |
*Healthy and diseased cows significantly different at p<0.05. **Significant at p<0.001. A/G ratio= Albumin to globulin ratio, ALT= Alanine transaminase, AST= Aspartate transaminase.
be due to the body immune response associated with Theileria infection (Singh et al., 2001). These results were in accordance with the results of Hussein et al. (2007), Ibrahim et al. (2009) and Devadevi et al. (2018) who reported hyperglobulinemia in cattle with theileriosis. In contrast, Kachhawa et al. (2016), Abubakar et al. (2019) reported hypoglobulenemia in theileriosis infected cattle.
There was significant (p<0.05) elevation in the total bilirubin level in the diseased group due to the marked increase in both direct and indirect bilirubin levels. The elevated indirect bilirubin values could be referred to increased destruction of infected erythrocytes. The elevated direct bilirubin values might be due to hepatic dysfunction and hemolysis in diseased cases. These results in harmony with that of Sandhu et al. (1998) and Singh et al. (2001).
Regarding liver enzymes, significant high (p<0.05) AST activities were observed in the sera of diseased cows. Similar results were recorded by Singh et al. (2001), Omer et al. 2003 and Kachhawa et al. (2016) who attributed the increased AST activity to the hepatic injury occurred during the disease. AST also exists in RBCs; therefore, the increased RBCs lysis occurred during the disease could be an important cause of elevated serum AST activity (Latimer, 2011).
ALT levels were not changed in the sera of diseased cows which is in agreement with the results of Lotfollahzadeh et al. (2012) and Devadevi et al. (2018) who reported no difference in the serum ALT activity during TT.
Concerning kidney functions, the obtained results in the present study revealed no significant changes in serum urea and creatinine levels in diseased animals. These findings are in accordance with those of Devadevi et al. (2018) and Abubakar et al. (2019) but disagree with the results of Ganguly et al. (2015) and Somu et al. (2017) who reported significant elevation of serum urea and creatinine concentrations in cattle with theileriosis.
In the current study, a significant (p<0.05) reduction in serum iron concentration was observed in diseased cows compared to healthy (Kumar and Malik, 1999; Omer et al., 2003). The decreased serum iron levels might be due to the inability of damaged hepatocytes in cattle infected with theileriosis to synthesize transferrin (Burtis and Ashwood, 1996). No significant changes were observed in the serum copper levels in the current study in cows with theileriosis as compared to healthy cows. These results were similar to those reported by Hussein et al. (2007) but differ from that of Kumar and Malik (1999) and Omer et al. (2003) who reported a significant reduction in the serum copper levels in theileriosis infected cattle.
Concerning lipid peroxidation, there was a significant increase (p<0.05) in the malondialdehyde activity in theileriosis affected cows as compared to healthy controls (Table 3). MDA is a lipid peroxidation end product, produced from polyunsaturated fatty acids oxidation. It is a profound and commonly used marker of lipid peroxidation (Moore and Roberts, 1998). In the present study, the significant elevation in MDA indicated occurrence of lipid peroxidation and oxidative stress at significant high levels in theileriosis affected cows that could be attributed to inability of antioxidant system to neutralize excessive free radicles generated during the disease course (Grewal et al., 2005; Nayak
Table 3: Oxidant-antioxidant alterations in healthy control and theileriosis affected cows.
| Parameter | Controls | Theileriosis affected cows |
P-value |
| MDA (nmol/ml) | 2.98±0.74 | 5.97±1.67 | 0.004* |
| TAC (mM/L) | 0.26±0.11 | 0.15±0.07 | 0.220 (NS) |
*Healthy and diseased cows significantly different at p<0.05. TAC= Total antioxidant capacity, MDA= Malondialdehyde.
et al. 2018; Singh et al., 2018). Free radicals cause oxidative damage to target molecules, such as lipids, nucleic acids and proteins. The red blood cells membrane is rich in polyunsaturated fatty acids which are the primary targets for oxidative damage induced by reactive metabolites (Davies and Goldberg, 1987) resulting in reduced membrane symmetry, increased membrane permeability, increased RBCs fragility and subsequently anemia (Saluja et al., 1999). These findings revealed that lipid peroxidation as consequence to oxidative stress in tropical theileriosis affected cattle plays a great role in RBCs destruction and accordingly anemia (Grewal et al., 2005).
The TAC measures the antioxidant capacity of all antioxidants, both enzymatic antioxidants and non-enzymatic antioxidants. Our results showed decreased TAC in diseased cows as compared to healthy (Guzel et al., 2008; Singh et al., 2018). The decreased TAC could be referred to reduction in the levels all antioxidants due to their exhaustion in neutralization of excessive free radicals generated in theileriosis affected cattle (Singh et al., 2018). Our data showed that T. annulata has a great effect on oxidant/antioxidant system resulting in a pronounced oxidative stress and exhausted anti-oxidant system in affected cattle which might be implicated in the pathogenesis and progression of bovine tropical theileriosis. Therefore, administration of antioxidants with anti-theilerial drugs as a supportive therapy may be beneficial for better and rapid recovery from tropical theileriosis.
Conclusion
Tropical theileriosis is a fatal disease with a characteristic clinical picture in which pyrexia, enlarged lymph nodes and respiratory distress are the main findings. Theileriosis induces significant hematologic alterations resulting in poor overall blood picture in which anemia is a main feature. Also, the disease causes significant biochemical changes pointed to liver function impairment during the disease course. Additionally, the findings proved that T. annulata has a great effect on oxidant/antioxidant system resulting in a pronounced oxidative stress. Anemia, impaired hepatic function and oxidative stress contribute to the pathogenesis, severity and prognosis of tropical theileriosis.
Acknowledgments
The authors are grateful to Hanan E. Saeed, assistant lecturer, department of clinical pathology, faculty of Veterinary Medicine, Beni-suef University for her help during the current study.
Conflict of interest
There is no conflict of interest.
authors contribution
Abdel-Hamied E., and Mahmoud M.M., participated in the design, planning and performing of the study. Aboelhadid S.M., and Arafa W., conducted the parasitological examinations and PCR assay. All authors participated in the discussion of results and writing the final manuscript.
References