Journal of Animal Health and Production
Research Article
Using of Real Time PCR as a Tool for Quantification of Sheep Pox Virus
Mohamed Samy Abousenna1*, Amal Abdelmenaem Mohamed1, Darwish Mahmoud Darwish1, Heba Abdelmenaem Khafagy1*, Shasha Fady Abdelmohsen1, Barghooth Waleed Mohamed 1, Nermeen Gouda Shafik1, Ayatollah Ibrahim Ibrahim2
1Central Laboratory for Evaluation of Veterinary Biologics (CLEVB), Cairo, Egypt; 2Veterinary Serum and Vaccine Research Institute (VSVRI), Cairo, Egypt.
Abstract | Sheep poxvirus (SPPV) is causing sheep pox clinical disease principally in sheep. In endemic areas, vaccination with live attenuated vaccines derived from SPPV provides protection from sheep pox disease. In current study, the evaluation of attenuated sheep pox virus vaccine was carried out in vitro by virus titration on tissue culture and in vivo by challenge test for vaccinated sheep to develop a rapid method to evaluate attenuated sheep pox virus vaccine using real time PCR compared to traditional method. Ten batches of commercial live attenuated sheep pox vaccines had been tested using the virus titration on tissue culture for quantification and real time PCR assay (qrt PCR) for identification and quantification of sheep pox virus in vaccine batches. It was found the minimal TCID50 that gave positive results of the qrt PCR for sheep pox virus was 1 TCID50/ sample. The efficacy of amplification was 1.99 with an R2 value of 0.991. The rt PCR gave results similar to tissue culture titration and statistical analysis showed a non significant difference (p > 0.05). These findings demonstrated the potential of rt PCR assay to supersede the traditional virus titration on tissue culture for identification and rapid evaluation of live attenuated sheep pox virus vaccines.
Keywords | Real time PCR, Sheep pox virus, Live attenuated vaccine, Vaccine evaluation, SPPV
Editor | Asghar Ali Kamboh, Sindh Agriculture University, Tandojam, Pakistan.
Received | December 17, 2019; Accepted | March 30, 2020; Published | April 10, 2020
*Correspondence | Abousenna M.S. and Heba A. Khafagy, Central Laboratory for Evaluation of Veterinary Biologics (CLEVB), Cairo, Egypt; Email: mohamedsamy2020@hotmail.com; dr.hebakhafgy@gmail.com
Citation | Abousenna MS, Amal AM, Darwish MD, Khafagy HA, Shasha FA, Barghooth WM, Shafik NG, Ibrahim AI (2020). Using of real time PCR as a tool for quantification of sheep pox virus. J. Anim. Health Prod. 8(2): 45-49.
DOI | http://dx.doi.org/10.14737/journal.jahp/2020/8.2.45.49
ISSN | 2308-2801
Copyright © 2020 Abousenna et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Capripox virus seems to be host specific with sheep poxvirus (SPPV) and goat pox virus (GTPV) causing clinical disease principally in sheep and goats, respectively (Diallo and Viljoen, 2007). Capripox virus is a large, complex, double-stranded DNA viruses of the genus Capripoxvirus, subfamily Chordopoxvirinae, family Poxviridae (Buller et al., 2005; Lamein et al., 2011). Sheep pox and goat pox are wide spread in Africa and Middle East (Diallo and Viljoen, 2007; Babiuk et al., 2008). It is a transboundary disease that impact negatively on livestock as it causes huge production losses due to damage to wool and hide, decrease in body weight, increase abortion rate and decrease in milk production (Yeruham et al., 2007). In susceptible population, the morbidity rate associated with sheep poxvirus can reach 100%, and the mortality rates can approach 50% in adults and 100% in young stock (Bhanuprakash et al., 2006). So, in Egypt vaccination with live attenuated sheep pox virus vaccine plays significant role to control the disease. This vaccine is routinely evaluated at Central Laboratory for Evaluation of Veterinary Biologies (CLEVB). Titration in vitro and challenge test in vivo are the standard and traditional methods for evaluation of the vaccine. A number of real-time PCR methods for the detection of capripoxviruses have recently been described (Balinsky et al., 2008; Bhanuprakash et al., 2008). Real-time PCR assays are quantitative, simpler and faster. It is also performed in closed vessel systems, low cost, avoids post PCR processing, thereby reducing risks from contaminations, and thus is suited ideally for rapid, specific and highly sensitive diagnosis during emergency cases (Valasek and Repa, 2005).
Table 1: Summary of the primer and labeled probe (FAM) q- PCR specifications of sheep pox virus.
|
Types of vaccine |
Forward (F) primer |
Reverse (R) primer sequence (5- 3) | Probe (P) sequence (5-3) | Type of fluorescence | Oligos location on GenBank sequence | PCR product size (bps) | Ann/Ext. temp. |
| Sheep Pox Virus | AAA ACG GTA TAT GGA ATA GAG TTG GAA | AAA TGA AAC CAA TGG ATG GGA TA | 5 -6FAM-TGG CTC ATA GAT TTC CT-MGB/NFQ3 | FAM | the P32 gene | 86bp | 60 °C |
This paper describes the development of a real time PCR assay using dual hybridization probes for the simultaneous detection of quality and quantity of the vaccinal strain and compare it with titration results and find relationship between titre of vaccinal strain by (infectivity) titration and by Real- time PCR.
Materials and Methods
Sheep pox virus
Reference sheep pox virus with a titer of 106 TCID50 (Rizkallah, 1994) was supplied by Strain Bank department at CLEVB. This virus was used as control positive for titration of sheep pox virus vaccine and for real time PCR standard curve.
Local commercial attenuated sheep pox virus vaccines
Ten batches of attenuated sheep pox virus vaccine were evaluated at CLEVB, Abbassia, Cairo. These batches had been evaluated around one year before for sterility, safety and potency.
African green monkey kidney (Vero) cells
Vero cells supplied by Strain Bank at CLEVB were used for (infectivity) titration of sheep pox virus vaccine.
Titration of local commercial attenuated sheep pox virus vaccines
Serial ten folds dilution of the local commercial attenuated sheep pox virus vaccines were prepared in Hank’s solution and inoculated into confluent tissue culture micro titer plate (VERO cell culture), 0.1 ml of diluted virus inoculums / plate and three plates/ each dilution (Rao and Malik, 1982). The plates were examined daily for 5 to 7 days for detection of cytopathic effect (CPE) of virus. The titer of virus was expressed as log 10 TCID50/ml of the original inoculation using the formula of Spearman (1908) and Karber (1931).
Real time PCR for quantification of attenuated sheep pox virus vaccine
The reference sheep pox virus contains 106 TCID50 was used for DNA extraction using manual (Qiagen DNeasy® Blood and Tissue Mini Kit, Qiagen, Germany) and robotic (Qiagen BioRobot Universal System, Qiagen, Germany) extraction techniques. The extracted DNA was then diluted serially 10-fold. Each dilution was tested in triplicate to determine the lower limit of the assay linear dynamic range by q-PCR. The extracted DNA was tested using the Quantifast Probe PCR Kit (Qiagen, Germany). The reactions were prepared in 20µl volumes with 2.0 _l of extracted viral nucleic acid, 20 µM of each primer (final concentration 900 nM) and 10 µM probe (final concentration 250 nM). Each reaction was performed in the thermal profile (Mx3005p, Stratagene) i.e., 1 × 50 oC for 2 min, 95 oC for 10 min, and 45 × 95 oC for 15 s and 60 oC for min (Bowden et al., 2008) followed by the reaction specific annealing/extension temperatures as specified (Table 1) for 60 s with optics ON. The cycle threshold number (Ct value) was determined to be the PCR cycle number at which the fluorescence of their action. Any reaction recorded Ct value, was considered positive and any reaction did not record Ct value, was considered negative; and used to establish standard curves. The quantitation and detection limit of each of the study’s real-time PCRs were determined by one run of each concentration for sheep pox live attenuated vaccines and determination of linear equation (Stubbs et al., 2012).
Data analysis
All 10 batches of live attenuated sheep pox virus vaccine were tested by real time PCR and (by infectivity) tissue culture titration and compared. The final results were analyzed by Wilcoxon’s test for matched pairs.
Results and Discussion
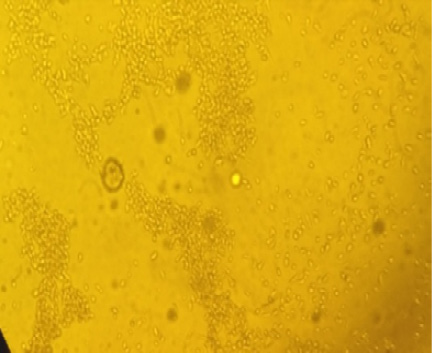
Characteristic CPE of sheep pox virus vaccine on Vero cell showing retraction of the cell membrane from surrounding cells, eventually rounding of cells and margination of the nuclear chromatin (inclusion bodies) as showed in Figures 1 and 2, The titrations of ten attenuated sheep pox virus vaccine batches on Vero cells shown in Table 2.
Table 2: Infectivity titration of different batches of attenuated sheep pox virus vaccine.
| Batch No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Reference sheep pox virus |
|
Titre (log 10 TCID50/ml) |
3 | 1.5 | 3.5 | 2.5* | 3 | 1 | 4 | 3.5 | 1 | 2.5 | 6 |
*Protective level should be not less than 2.5 log 10 TCID50
Each dilution of reference sheep pox virus was running at the same reaction Figures 3 and 4. The minimal concentration of sheep pox virus gave positive result of 101 TCID50 as shown in Table 3.
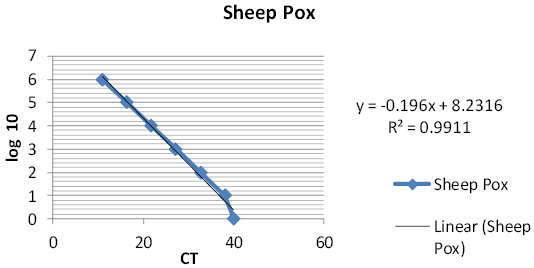
The linear equation, the mean CT values and r-squared value of the obtained standard curves of reference sheep pox virus were summarized in Figures 3, 4 and Table 3.
The ten batches of live attenuated sheep pox virus vaccine were titrated by qrt- PCR and gave results similar to tissue culture titration and there was no significance difference as shown by the p-value i.e., 0.3662 (Table 4).
Table 3: Summary of the mean (cycle threshold) CT values, the linear equations and the R-squared values of the real-time PCR for sheep pox virus.
| Virus Dilution | *(CT values ) |
|
106 |
10.90 |
|
105 |
16.33 |
|
104 |
21.761 |
|
103 |
27.19 |
|
102 |
32.619 |
| 10 | 38.04 |
| Linear equation | Y= -0.196X+8.2316 |
|
R-squared |
0.9911 |
* Cycle threshold.
Table 4: Comparison between the q-PCR titer and (infectivity) titration on tissue culture for attenuated sheep pox virus vaccine batches.
| Sheep Pox Virus | ||||
| Batch no. | Ct | Equation | qrt-PCR titer | Infectivity titration |
| 1 | 27.2 |
Y= -0.196X+ 8.2316 |
2.9 | 3 |
| 2 | 33.45 | 1.67 | 1.5 | |
| 3 | 24.65 | 3.4 | 3.5 | |
| 4 | 29.12 | 2.52 | 2.5* | |
| 5 | 26.14 | 3.1 | 3 | |
| 6 | 35.25 | 1.3 | 1 | |
| 7 | 21.36 | 4.04 | 4 | |
| 8 | 24.41 | 3.44 | 3.5 | |
| 9 | 36,09 | 1.16 | 1 | |
| 10 | 28.5 | 2.64 | 2.5 | |
|
There is no significant difference The p-value is 0.3662 |
||||
Ct: cycle threshold; qrt- PCR: real time polymerase chain reaction; *Protective level should be not less than 2.5 log 10 TCID50
The aim of this study was to further evaluate the most suitable real-time PCR assay for the detection of capri pox viral DNA and adapt it for high-throughput testing. Two previously published real-time PCR assays (Balinsky et al., 2008; Bowden et al., 2008) were selected and evaluated using various criteria including simplicity, sensitivity and specificity than titration and challenge test. Titration of ten batches of live attenuated sheep pox virus vaccine was inoculated on confluent Vero cell cultures according to Rao and Malik (1982). Cytopathic change began showing retraction of the cell membrane from surrounding cells, eventually rounding of cells and margination of the nuclear chromatin (inclusion bodies) as shown in Figures 1 and 2. The titer was calculated by using the formula of Spearman (1908) and Karber (1931). It was found that seven batches gave titre 10 2.5TCID50/ml or more while three batches (No. 2, 6, 9) gave titre 1.5, 1, 1 log 10TCID50 respectively (Table 2). These results were agreed with (OIE, 2010) that recommended the titer of attenuated sheep pox virus vaccines on vero cell culture as 10 2.5 TCID50/ml.
Using the commercially available Qiagen QuantiFastTM Probe PCR Kit, Bowden et al. (2008) reported that real-time PCR assay had ability to identify and quantify sheep pox virus isolates and the attenuated sheep pox virus vaccine. The sensitivity (minimal TCID50 that gave positive results) of the qrt PCR for sheep pox virus is 1 TCID50/ sample, the efficacy of amplification was 1.99 with an R2 value of 0.991. Balinsky et al., 2008 recorded sensitivity assay ranged between 1.6 and 15.8 TCID50 with amplification efficacy 1.99 and an R2 value of 0.99 for SPPV using rt-PCR taqman labeled probe while the detection limit of Pestova et al. (2018) was as low as 0.1 TCD50 lg/ml for the LSDV field strain Russia 2015 using Rotor-Gene Q (Qiagen) platform.
The standard curve and linear equation were established to determine the unknown TCID50 for the new entry vaccine batch within 2 hrs.
The comparison between q-PCR titer and (infectivity) titration on Vero cells for ten batches of live attenuated sheep pox virus vaccine showed no significant difference between the results. The p-value was 0.3662 (p > 0.05, using Wilcoxon’s test for matched pairs). This agreed with Sayed et al. (2018) who demonstrated the comparison between qrt-PCR titer and bacteriological count for live MG batches (10 batches for each types F-strain and Ts-11 vaccines). The P= 0.02 (0.01-0.05) value was 0.01 and 0.012 for F-strain and Ts-11 respectively that’s no difference in significance between the two test.
In conclusion, the real time PCR assay is simple, sensitive and specific thus enabling the rapid, high-throughput testing for sheep pox virus in live attenuated vaccines, this method has the potential to supersede the traditional virus titration on tissue culture for identification and rapid evaluation of live attenuated sheep pox virus vaccines.
Authors Contribution
All authors contributed according to their assigned tasks and approved the final manuscript.
Conflict of interest
The authors have declared no conflict of interest.
References