Journal of Animal Health and Production
Review Article
The In Ovo Feeding Technique as a Recent Aspect of Poultry Farming
Karim El-Sabrout1*, Sohail Ahmad2, Ahmed El-Deek1
1Department of Poultry Production, Faculty of Agriculture (El-Shatby), Alexandria University, Alexandria, Egypt; 2Department of Poultry Production, University of Veterinary and Animal Sciences, Lahore, Pakistan.
Abstract | The incubation period of chicken eggs is 21 days; for meat-type birds, this represents approximately 60% of their life. In most cases, chicks are shifted from the hatchery when the majority (approximately 95%) of the chicks have hatched. This period time, added with the time required to transport the chicks to rearing farms, means that chicks can be deprived of water and feed for 2-3 days. Due to the specificity of the embryogenesis process and the unique avian eggshell structure, the development of the embryo could be directly manipulated via a small window that is cut into the egg-shell to allow access to the embryo. This ability has been used to develop the in ovo technique as a biotechnology approach, i.e. direct injection of bioactive and nutritional substances during the incubation process. Recent studies have shown major developments in the utilisation of this technique for early feeding and supply of carbohydrates and amino acids (more than 2.5% weight enhancement for broilers) or vaccination (Newcastle, Mareks Disease, with more than 2% immunity enhancement) in several commercial poultry farms particularly in Europe, United States and Brazil. Additionally, the injection of IGF type I (100 ng/egg from human origin) on the 3rd day of incubation increased the efficiency of growth and development of muscle during the first weeks of life resulting in more than 12% weight enhancement for broiler. The amniotic site has been considered the most effective for supplements injection, while the 18th day of chicken egg incubation is the most effective time. This review integrates recent aspects that could influence traits of interest for in ovo feeding in the poultry industry.
Keywords | Chicken Embryo; Feeding; Hormone; Immunity; In Ovo.
Received | July 20, 2019; Accepted | September 02, 2019; Published | November 07, 2019
*Correspondence | Karim El-Sabrout, Department of Poultry Production, Faculty of Agriculture (El-Shatby), Alexandria University, Alexandria, Egypt; Email: kareem.badr@alexu.edu.eg
Citation | El-Sabrout K, Ahmad S, El-Deek A (2019). The In Ovo Feeding Technique as a Recent Aspect of Poultry Farming. J. Anim. Health Prod. 7(4): 126-130.
DOI | http://dx.doi.org/10.17582/journal.jahp/2019/7.4.126.130
ISSN | 2308-2801
Copyright © 2019 El-Sabrout et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Science and technology have made a significant contribution to create new aspects that have been used in the improvement of the poultry industry. The hen egg is composed of water (74%), proteins (12.8%), lipids (11.8%), and small amounts of carbohydrates and minerals (approximately 1%) (Guerrero-Legarreta, 2010). Although the natural and unique chemical composition of an egg makes it a principle and complete source of nutrition for the embryo, in ovo technology could provide the embryo with suitable nutrients that may enhance its development. Under commercial incubation, chicken eggs hatch over 24 to 48 h period in incubators (Zhong et al., 2018). During this time, already hatched chicks are left without any available external food. To realise the maximum genetic potential of meat-type birds, a good start is required at the hatchery. Several factors affect the quality of chicks in hatcheries, one of them is chicks hatching window. In most cases, chicks are shifted from the hatchery when the majority (95%) of the chicks have hatched. This period time, added with the time required to transport the chicks to rearing farms, means that chicks could be starved of water and feed for up to 48-72 h (Kadam et al., 2013). During this time, residual yolks containing fats and proteins are the only sources for chick maintenance and growth (Sklan et al., 2000). Recent studies have revealed the significance of early nutrition in the overall health and performance of commercial broilers. Generally, residual yolk is considered sufficient during the first 72 h, but growth is largely dependent upon feed consumption and the nutrients present in the yolk. These nutrients are utilised by the chick during the fasting period; however, they are insufficient to fulfil the requirements for the growth and maintenance of broiler chicks. It is commonly reported that delayed feeding has a negative effect on growth, mortality, market age, and overall meat quality traits.
Using the in ovo technique, nutrients are supplemented through the amniotic sac (air chamber) of the embryo at a later stage of development, particularly at day 18 of egg incubation, to provide a continuous supply of essential nutrients during the first days of the post-hatch period (Kucharska-Gaca et al., 2017). These are important to support the development of thermoregulation, the digestive system, and feather development. However, enzymatic activity in the embryo greatly changes after hatching, particularly during the first 10 days, which affects nutrient absorption and digestive capacity in birds (Kadam et al., 2013). For example, carbohydrate and protein absorption is reduced on the day of hatch and increases during the first four days post-hatch. In contrast, the absorption of fatty acids increases at hatching and remains high during the first four days post-hatching (Cardeal et al., 2015).
During the development of the immune system, the nutritional status of the developing embryo is crucial to maintain immunity (Bakyaraj et al., 2012). This is the reason that severe deficiency of any nutrient during the development of the immune system and its responses negatively affects immune competence.
The In Ovo Technique in Poultry
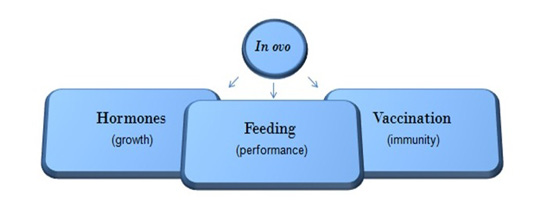
The use of the in ovo technique in poultry was first reported several years ago (Kadam et al., 2013); however, recent studies have shown major developments in the utilisation of this technique for early feeding, hormone supplementation or vaccination in several commercial poultry farms particularly in Europe, United States and Brazil (Figure 1). With the in ovo technique, bioactive substances can be administered directly to the incubating eggs. These include carbohydrates, proteins (amino acids (AAs)), fatty acids, minerals, vitamins and growth promoters (Bednarczyk, 2016). This technique enables the delivery of substances to the developing embryo, which can compensate for the starvation period of newly hatched chicks and facilitate a healthy gastrointestinal tract (GIT), its microbiome and ultimately enhance post-hatch performance (Kucharska-Gaca et al., 2017).
In commercial poultry production systems, newly hatched chicks are prevented from feeding (almost 48-72 h) to achieve complete utilisation of the residual yolk (Kadam et al., 2013). This delay depends largely on hatching time and is only applicable when there is an ideal, and short, hatching window (high uniformity). In a recent study, decreased growth rate was noted in commercial broiler chicks subjected to delayed feeding compared to the birds fed immediately after hatch (Roto et al., 2016). AA and carbohydrate injection promoted the activity of digestive enzymes and facilitated intestinal maturation (Kadam et al., 2013). Uni and Ferket (2004) was also revealed that in ovo injection at 18 days of incubation with 1 mL of saline containing carbohydrate resulted in a significant increase in jejunal villus height by over 45% only 48 h after injection.
With regards to early stimulation of the gastrointestinal tract, enhanced growth rate (approximately 10% weight gain) has been observed (Uni and Ferket, 2004). Similarly, the administration of vitamins has a positive influence on the growth rate (more than 330g weight gain) with a higher feed conversion ratio of 0.4 (FCR) on average than control (Selim et al., 2012). Furthermore, in ovo injection of different bioactive substances not only enhanced embryonic growth (2-3%) but impacted post-hatch growth (more than 5%), intestinal health (Ohta et al., 1999; Bednarczyk et al., 2011), and meat quality (Maiorano et al., 2012), increased digestive enzyme activity (Pruszynska-Oszmalek et al., 2015) and facilitated the development of the immune system (Madej et al., 2015; Bednarczyk, 2016).
In Ovo Feeding and Growth/ Immune Response
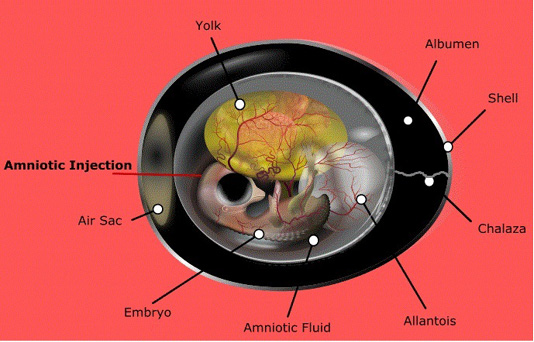
Literature regarding the in ovo technique supports its efficacy in accelerating enteric growth and nutrient digestion in young chicks (Ferket, 2012). Different methods have been applied with various materials for promoting the growth and productivity of commercial poultry. Generally, there are five possible sites of in ovo injection: the air cell, the embryo’s body itself, the amniotic fluid, the allantoic membrane, and the yolk sac (Saeed et al., 2019), however, the amniotic is considered as the most effective site for injection and directly influencing the developing embryo (Kucharska-Gaca et al., 2017) (Figure 2). Nevertheless, the volume and concentration of the injected material are largely dependent upon bird type, egg size, injection site, incubation system and type of material. In this regard, both manual and automatic methods have proven to be effective, while commercial applications require automation (Peebles, 2018).
Recent research on in ovo techniques has indicated that different bioactive substances have a positive influence on the gut health and the overall productivity of birds. Early nutrition process is an effective application to feed embryos and prepare chicks with healthy gut, beneficial microbiota, enhanced immunity, and overall improved growth performance (Jha et al., 2019). The injection materials have included vaccines, drugs, hormones and supplemental nutrients (Kadam et al., 2013). Some of these materials have proven to be viable for enhancing immunity, growth and post-hatch performance. Vaccines approved for the in ovo technique include those for Marek’s disease, Newcastle disease, fowl pox, infectious bursal disease and coccidiosis (Peebles, 2018). Bhanja et al. (2014) concluded that arginine and threonine enhanced the expression of growth-related genes, while threonine and methionine+cysteine modulated expression of immune genes in broiler chickens.
In contrast, injection of insulin-like growth factor (IGF-I) enhanced muscle development during the first week of bird life (Kucharska-Gaca et al., 2017). Kocamis (1998) reported that the injection of IGF type I (100 ng/egg IGF of human origin) on the 3rd day of incubation increased the efficiency of growth and development of the muscle during the first weeks of life. This hormone positively influenced the development of muscle tissue (approximately 10% breast weight gain) with a significantly greater gain in weight (12.3%) reported in broilers.
Additionally, numerous studies have reported that the administration of prebiotics, probiotics, and symbiotics during the 18th day of incubation significantly influenced embryonic growth (2-3% increase) and post-hatch performance and help fight intestinal bacterial infections. Moreover, folic acid, albumen proteins and different AAs (arginine, histidine, lysine, and threonine) are effective and influence post-hatch growth (more than 10% increase) (Bhanja et al., 2014). Uni et al. (2005) reported that in ovo feeding solutions containing carbohydrates and protein increased broiler chick weights by 3-7% over controls, depending upon the breeder hen age and in ovo feed composition. However, vitamins C and E are considered the most effective at enhancing immunity, whilst carbohydrates can be used to increase glycogen storage. Creatine can be used to enhance muscle development. Microminerals and vitamin D3 have been shown to enhance skeletal development and improve bone strength.
During the post-hatch stage, chicks undergo a series of stresses related to their surrounding environment such as artificial light, unfamiliar sounds and odours, restricted feeding, temperature alterations, lack of retreat space and diseases (Morgan and Tromborg, 2007). Good management during this early stage is mandatory for ideal growth and survivability in the future (Siegel, 1995; Klasing, 1998). From a nutritional point of view, biologically active substrates such as carbohydrates, AAs, enzymes, and cofactors are required to facilitate clonal proliferation of antigen-driven lymphocytes and synthesis of cytokines, lysozymes, and immunoglobulins. Furthermore, AAs could be helpful for the development of skeletal muscles and, in a healthy state, for promoting hepatic AA uptake and to increase protein synthesis.
In ovo feeding is the only technique that ensures a direct supply of nutrients and cofactors during the developing phase of an embryo and has a positive effect on chick weight (2-3%), post-hatch growth (more than 10%), immunity and market age. A preliminary study revealed that administration of vitamin E at 0.25-0.50 IU during the late embryonic stage improved post-hatch growth and humoral immunity. However, the provision of linoleic acid increases cell-mediated immunity (CMI) (approximately 20%) in commercial broilers (Bakyaraj et al., 2012). Similarly, it was reported that injecting ω-3 fatty acids, ω-6 fatty acids and conjugated linoleic acid increased production performance, antibody response and cell-mediated responses, respectively (Selvaraj and Cherian, 2004). The provision of ω-3 fatty acids of marine origin has proven to reduce interleukin-12 (IL-12) and interferon γ (IFN-γ) release, resulting in a shift in the response of T-helper 1 cells (predominantly involved in the cell-mediated immune response) towards T-helper 2 cells (predominantly associated with the humoral response). In ovo supplementation of AAs, fatty acids, trace elements and vitamins on the 18th day of incubation at the broader end of the egg using a 25 mm needle had a positive impact on post-hatch growth and immunity (Bakyaraj et al., 2012).
Administration of In Ovo Nutrients
Administration of AA such as methionine, arginine, lysine, leucine, and isoleucine promote CMI, while methionine, threonine, glycine, valine and serine increase humoral immunity (more than 35% in thymus) (Bakyaraj et al., 2012). The mineral-base and immunological responses in developing embryos are still unclear; however, selenium and zinc have shown positive effects on humoral and CMI (more than 50% and 35% increase, respectively). Haq et al. (2017) indicated that supplementation of chromium individually or in combination with vitamin C/vitamin E has several benefits on feed consumption, feed conversion ratio, and body weight. Deficiencies of pantothenic acid and riboflavin negatively impact the growth and the humoral immunity of chicken embryo, therefore, the supplementation of these critical vitamins is very important for the development of the embryo (Kadam et al., 2013). Additionally, administration of vitamins such as vitamin C (ascorbic acid) and vitamin E (alpha-tocopherol) during the 18th day of incubation typically results in increased body weight and immunity (Kadam et al., 2013). Vitamin B6 deficiency has a negative effect on pyridoxal phosphatase function, which facilitates the transformation of AA for protein synthesis required for the immune response. Administration of sucrose, fructose and grape seed extract (GSE) during the 18th day of incubation resulted in better hatchability, whereas a positive correlation was also reported in day-old chick weights with injection of AAs, glucose, and magnesium (Kucharska-Gaca et al., 2017). Whether early feeding of these nutrients (in ovo supplementation) can help in modulating immunity for post-hatch chicks has been investigated. Bakyaraj et al. (2012) found that in ovo injection of some critical AA, fatty acids, vitamins, and trace elements on early post-hatch growth impacted the development of lymphoid organs (thymus, bursa, and spleen) (20-50% increase) and immune parameters (1-3% increase) in broiler chickens.
CONCLUSION
The objective of this review is to address the in ovo technique as a possible approach that can contribute to enhancing the productivity, immunity and decreasing the hatch window as well as to increasing the uniformity of post-hatch chicks. The chicks early feeding with supplements such as amino acids, hormones and vaccines has been shown to improve total digestive tract development, post-hatch growth, and immune- response. However, the amniotic site has been considered to be the most effective site for supplements injection, while the 18th day of chicken eggs incubation has been considered to be the most effective time. Future study is likely to focus progressively on how nutrients introduced into the egg affect genes and if nutrients can be combined with commercial vaccine diluents during the in ovo vaccination to become more acceptable and effect. In addition, new nutrients with multiple functions (growth and immune stimulants) such as organic acids, fenugreek, nutraceutical, and even bee venom should be evaluated using in ovo technique.
Acknowledgements
The author is grateful to the Egyptian Knowledge Bank (https://www.ekb.eg/web/ guest/home) for its support.
Conflict of interest
The authors declare that there is no known conflict of interest associated with this publication.
Author contributions
KE collected and wrote this review. SA and AE revised it.
Animal welfare statement
The authors confirm that the ethical policies of the journal, as noted on the journal’s author guidelines page, have been adhered to “No ethical approval was required as this is a review article with no original research data”.
REFERENCES