The Journal of Advances in Parasitology
Research Article
Effect of Environmental Factors on the Intensity of Nematode Infection in Marine Fishes of Visakhapatnam, A.P, India
P. Rosina George1*, B. Bharatha Lakshmi2
1Assistant Professor, Department of Zoology, St. Joseph’s College for Women (A), Visakhapatnam, Andhra Pradesh; 2Professor, Department of Zoology, Andhra University, Visakhapatnam, Andhra Pradesh, India.
Abstract | Environmental factors are of critical significance influencing the parasitic infection in fish and the subsequent expression of pathogenic potential. It has been seen that the nematode infection relates to the environmental factors like temperature and salinity. These two important parameters evidently influence the intensity of nematode infection in the marine fish community. Twenty-eight nematode parasites were recorded during the study in 112 marine fish species examined. Hundred and ninety-eight fish (8% of total sample) were infected with nematodes among 2500 fish specimens examined. The prevalence of nematode parasites was high in summer season followed by rainy season and winter season in all the three years (January 2007-December 2009). Based on the data given by the National Institute of Oceanography (N I O), Visakhapatnam, that the marine climate particularly of the coastal regions there are two peaks of high temperatures in every year recorded from 2007 to 2009. The intensity of nematode infections was also recorded high in these peak periods. This is an indication that the high temperature of the coastal marine environment plays an important role for the intensity of nematode infection to the marine fish, whereas during this period (Jan 2007 to Dec 2009) there was not much variation in salinity in the horizontal depth stratification ranging from 5 to 90 meters depth. The purpose of this study was to find out the effect of temperature and salinity on the intensity of nematode infection in edible marine fishes of Visakhapatnam.
Keywords | Nematode parasites, Temperature, Salinity, Intensity, Environmental factors
Editor | Muhammad Imran Rashid, Department of Parasitology, University of Veterinary and Animal Sciences, Lahore, Pakistan.
Received | May 17, 2021; Accepted | June 24, 2021; Published | June 30, 2021
*Correspondence | P Rosina George. Assistant Professor, Department of Zoology, St. Joseph’s College for Women (A), Visakhapatnam, Andhra Pradesh; Email: rosina.rovic@gmail.com
Citation | George PR, Lakshmi BB (2021). Effect of environmental factors on the intensity of nematode infection in marine fishes of Visakhapatnam, A.P, india. J. Adv. Parasitol. 8(2): 20-25.
DOI | http://dx.doi.org/10.17582/journal.jap/2021/8.2.20.25
ISSN | 2311-4096
Copyright © 2021 George et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Environmental factors are of crucial importance influencing the parasitic infection in fish and the subsequent expression of pathogenic potential. Environmental changes such as water temperature, salinity or dissolved oxygen can reduce the effectiveness of the fish to combat diseases and so increase the risk of parasitism (Dogiel, 1958; Kennedy, 1972; Ribeline and Migaki, 1975). It has been noticed that the nematode infection relates to the environmental factors like temperature and salinity. These two significant parameters distinctly influence the intensity of nematode infection in the marine fish community. This has been observed in the marine environment that the intensity of nematode infection to the fish is higher during the periods of high temperature and less infection during the periods of low temperatures.
In Visakhapatnam although ample literature is available on species of nematode parasites infecting the fishes however the studies handling environmental factors on the intensity of nematode infection in fish isn’t done. Eaton et al. (1982) studied nematode infection in the northern anchovy Engraulis mordex from San Francisco Bay where he found nematode Contracaecum sp larvae found in increased its parasitic infection as water temperature increased. In a similar way Haseen Fatima (1988) reported the seasonal variation of nematodes of some edible fishes of Karachi coast where she observed that the maximum infection was in June and minimum in January and December. Chubb (1982) emphasized that water temperature acts directly on the helminthes or indirectly through the host behaviour, especially feeding behaviour and metabolism. In the present study, it may be one of the reasons of leading to higher parasitic infection in the fishes in summer. The intensity of infection was highest in summer, and lowest in winter in the studies on seasonal variation of the nematode Camallanus anabantis in the fish Anabas testudineus in Loktak Lake, Manipur, India (Ranibala et al., 2013). Sheema et al. (2015) and Ritika et al. (2012) have reported the plenitude of helminths increment with the rising temperature in summer and delayed down during winter. The development of intermediate hosts of helminths during summer season additionally prompts better accessibility of infective stages bringing about higher helminths predominance in summer (Khurshid and Ahmad, 2012). Kalse (2014) revealed during examinations on population dynamics of nematode parasite in Mastacembellus armatus that parasitization of fishes relies upon the environmental factors. Recently, Dr. Shalini Roy and Neelam Kumari (2020) indicated that ecological factors play an important role in parasitic infestation.
Salinity is another important parameter for the survival of animals in the marine environment and regulates their distribution. Parasites in the fish intestines appear to be unaffected by changing water salinities, as the osmolarity in the intestines stays nearly constant (Moller, 1978). Measures (1996) discovered temperature and water salinity impact in the development of Pseudoterranova decipiens eggs. The purpose of this study is to find out the effect of temperature and salinity on the intensity of nematode infection in edible marine fishes of Visakhapatnam. The current investigation gives a data and attention to the pesetarians, which sort of palatable fish ought to be chosen in a specific season.
MATERIALS AND METHODS
Fish samples for the present study were collected from the trawlers operated from the Visakhapatnam coast and were also collected from the local fish markets (most come from Raiwada, Thandava and Mehadrigedda reservoirs) from January 2007 to December 2009. Fishes were brought intact to laboratory and washed with tap water and kept in the freezer till further analyses. Fishes were carefully examined for parasites externally and also internally. 2ml of 60+10 C of habitat water when added to an active nematode, which influences to restrict the movement. In a similar way 400C +10 increases the activity of the nematode parasites. Specimens were fixed by placing them in a beaker containing warm 70% alcohol for ten minutes. They become straight and extended. Afterwards they were transferred to 70% alcohol with 5% glycerine for storage in sealed containers.
The temporary mounts of nematodes were prepared after clearing the cuticular structures such as striations and papillae with the help of either glycerine or lactophenol. After getting sufficient transparency they were examined with the microscope for studying the internal organs of the specimen for their identification. The diagrams were drawn with the aid of camera lucida.
The following terms was used to describe the parasitic infestations (Margolis et al., 1982):
1. Prevalence (%) = Total number of infected fishes x 100
Total number of hosts examined
2. Mean intensity = Total no. of parasites collected
Total no of infected host examined
3. Relative density or Abundance =
Total no. of parasites recovered
Total no. of host examined
Statistical analysis
Data were computed using MS Excel statistical analysis software on Microsoft 10 package.
RESULTS AND DISCUSSIONS
Temperature and salinity are two relevant environmental parameters that affect the biological processes of animals in marine environment. Twenty-eight nematode parasites were recorded during the study in 112 marine fish species examined. Hundred and ninety-eight fish (8% of total sample) were infected with nematodes among 2500 fish specimens examined. On the basis of the three years data, it was found that the temperature is an important parameter which influences the intensity of infection. The present study describes the effect of seasons on total nematode population from the hosts collected during three annual cycles, 2007-2009. Each annual cycle comprises (1) Rainy Season (July to October), (2) Winter Season (November to February), (3) Summer Season (March to June). From the Table 1, it indicates that in three years, the incidence of infection was moderate in winter and has risen considerably in rainy season and reached its peak in summer season. As the temperature increases, the intensity of nematode
Table 1: Prevalence and Mean Intensity of nematode infection recorded season wise in marine fishes of Visakhapatnam coast (2007-2009)
| Year | Season | No. of examined fishes | No. of infected fishes | Total parasites | Prevalence (%) |
Mean intensity
+ S.D* |
| 2007 |
Rainy Winter Summer |
290 239 291 |
19 2 62 |
41 5 98 |
6.5 0.8 21.3 |
2.2 + 1.09 2.5 + 1.25 1.5 + 0.94 |
| 2008 |
Rainy Winter Summer |
273 270 272 |
14 1 31 |
21 1 42 |
5.1 0.3 11.3 |
1.5 + 0.42 1 + 0.5 1.3 + 0.21 |
| 2009 |
Rainy Winter Summer |
273 272 275 |
23 5 41 |
30 6 49 |
8.4 1.8 14.9 |
1.3 + 0.14 1.2 + 0.64 1.1 + 0.05 |
Rainy Season (July to October), Winter Season (November to February), Summer Season (March to June). * + S.D = + Standard Deviation
infection increases when compared to the low temperatures. This indicates that in summer season nematode infection is more than the winter season. The present study correlates with the view of Kumari Gautam et al. (2018) who suggested that the infestation rate was higher in summer than in other seasons. In the present study, Prevalence of infection was observed highest in the month of May in all three years (2007-2009), when the average sea water temperature was 30.060C. In the present study, incidence of nematode parasitism in fish was highest in summer (March-June) and lowest prevalence was found in winter (November-February) whereas the mean intensity was highest in winter (November-February) and lowest mean intensity was found in summer (March-June) (Figure 1). From the total period of 3 years the nematode parasites exhibit a marked seasonal incidence, with peak infestation recorded among Upeneus vittatus with 62.5% prevalence, Thryssocles mystax (50%), Upeneus sulphureus (50%) and Saurida undosquamis (46.6%). %) (Figure 2) with Contracaecum occurring in summer i.e., from March to June. Ismen and Bingel (1999) observed the nematode infection in the Whiting Merlangius merlangus euxinus off Turkish coast where the nematode parasite Hysterothylacium aduncum showed highest prevalence and intensity in the warm season (July-Aug) than in the colder period (Jan-Feb). Steinauer and Font (2003) described the seasonal dynamics of helminths of blue gill fish Lepomis macrochirus where he found the nematode Camallanus oxycephalus exhibited a distinct seasonal abundance and prevalence which peaked in summer. Similarly, Vincent and Font (2003) reported that the nematode Camallanus cotti showed higher prevalence and mean abundance in summer than in winter.
Effect of Temperature on the intensity of infection in marine fishes
Based on the data given by the National Institute of Oceanography (N I O), Visakhapatnam, that the marine environment especially of the coastal regions there are two
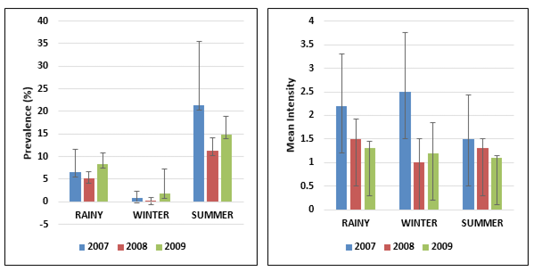
Figure 1: Error bars represent the standard deviation of the Prevalence and Mean Intensity of infection season wise from 2007-2009
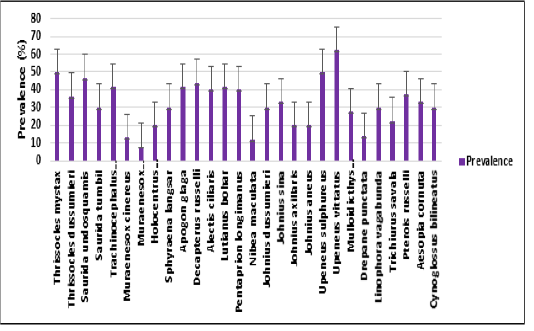
Figure 2: Error bars represent the standard deviation of the prevalence of infection in each fish species in a total period of 3 years 2007 to 2009
peaks of high temperatures in each year recorded from 2007 to 2009. The intensity of nematode infections was also recorded high in these peak periods. This is an indication that the high temperature of the coastal marine environment plays an important role for the intensity of nematode infection to the marine fish. On the basis of the recorded evidence the effect of temperature is directly proportional to the intensity of activity of a nematode parasite which influences the intensity of infection. This factor has been observed clearly in the marine environment where the intensity of nematode infection to the fish is higher during the periods of high temperature and less infection during the periods of low temperatures. Caspeta-Mandujano and Mejia-Mojica in 2004 reported that the nematode Rhabdochona canadensis infection in a fish Notropis boucardi was highest in prevalence and mean intensity in May and low in January. The present study holds true with the views of Genc et al. (2005) and Puinyabati et al. (2010) who suggested that the parasite infection showed seasonal variations with the highest prevalence in summer season. They also reported that the warm water conditions resulted in an unusually heavy infestation of nematodes.
In the year 2007, two peaks of high temperatures were recorded. The first peak of high temperatures 30.040C and 29.560C were recorded during the month of May and June respectively. In these two months the high intensity of nematode infection among the fish is recorded as 62.8% and 31.2% respectively (Figure 3). Thryssocles mystax and Upeneus sulphureus were among the infected fishes who were infected by the nematode parasite Contracaecum vittate. The second peak of high temperatures i.e 29.060C and 29.440C were recorded during the month of September and October respectively (Figure 3). In these two months the intensity of nematode infection in the fish is also high as 16.6% and 24.6% respectively. Mulloidicthys auriflamma and Alectis indica were infected by the nematode parasite Rhabdochona sp and Raphidascaris diadonis.
In the year 2008 the first peak of high temperatures 30.0C and 30.120C were recorded during the month of May and June respectively (Figure 3). In these two months the high intensity of nematode infection among the fish is recorded as 22.8% and 15.2% respectively. Saurida undosquamis and Johnius sina were among the infected fishes who got infected high by the nematode parasite Contracaecum sp and Contracaecum vittati. The second peak of high temperatures 28.990C and 28.090C were recorded during the month of September and October respectively (Figure 3). In these months the intensity of nematode infection in the fish is also high as 5.7% and 9% respectively. Pentaprion longimanus and Trachinocephalus myops were infected by the nematode parasite Contracaecum vittati and Contracaecum sp.
In the year 2009 the first peak of high temperatures 30.160C and 29.240C were recorded during the month of May and June respectively (Figure 3). In these two months the high intensity of nematode infection among the fish is recorded as 24.2% and 18% respectively. Among the infected fish Upeneus vittatus, Saurida tumbil and Trichiurus savala were infected high by the nematode parasite Contracaecum vittati, Indocucullanus arabiansae and Camallanus savala n.sp. The second peak of high temperatures 29.550C and 29.570C were recorded during the month of September and October respectively (Figure 3). In these months the intensity of nematode infection in the fish is also high as 11.7% and 14.2% respectively. Among the infected fish Sphyraena langsar, Thyrissocles dussumieri and Cynoglossus bilineatus were infected high by the nematode parasite Paranisakis and Contracaecum sp.
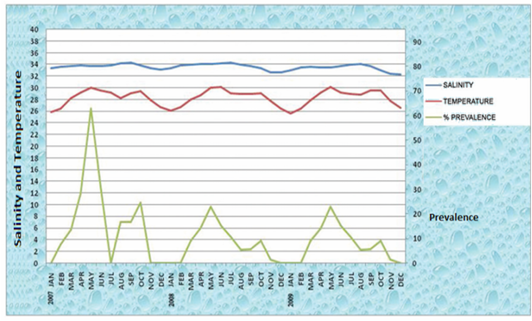
Figure 3: Comparison of Salinity, Temperature with Prevalence of infection in a total period of 3 years 2007-2009
The low temperatures were recorded as 25.842720C, 27.858140C and 26.720410C during the months of January, November and December respectively in the year 2007. The percentage of infection is nil in these three months. In other months less intensity of infection was observed (Figure 3).
The low temperatures were recorded as 26.111390C, 26.6950C and 26.409770C during the months of January, February and December respectively in the year 2008. The percentage of infection is nil in these three months. In other months less intensity of infection was observed (Figure 3).
The low temperatures were recorded as 25.636130C and 26.624840C during the months of January and December respectively in the year 2009. In these two months the percentage of infection is nil. In other months less intensity of infection was observed (Figure 3).
Effect of Salinity on the intensity of infection in marine fishes
Salinity has been shown to be a persuasive environmental factor for parasitism and illnesses in brackish and estuarine environment and under normal to increased salinities, conditions are more favorable for the parasite (Fengyang and Poulin, 2011; Studer and Poulin, 2012). In the present study data has been collected to examine the influence of salinity on infection and distribution of nematode parasites in the coastal marine waters of Visakhapatnam. According to the information of the operators of the trawlers and fishing boats, who operate between 5 to 90 meters depth. Information on salinity in the coastal waters of Visakhapatnam coast has been obtained from National Institute of Oceanography (N I O) for a period of three years i.e from January 2007 to December 2009. There was not much variation in salinity during this period in the horizontal depth stratification . Salinity ranged between 33.407o/oo to 34.702 o/oo. On the basis of the results of the present work the salinity in the marine environment showed negative impact on the intensity of nematode infection (Figure 3). This has been confirmed as the salinity variations are negligible in three years in the depths of 5 to 90 meters of Visakhapatnam coast. But according to Kirk et al. (2000) the larva of nematode parasite Anguillicola crassus infects minimum with increased salinity to the eel.
The results of this study imply that the intensity of infection in relation to the effect of temperature in the natural environment was found, that the increased temperature increases the intensity of infection. Salinity factor was not having impact on the intensity of infection. These results may show that even slight changes in seasonal temperatures might affect the phenology of parasites conceivably prompting extensive results that need additional examinations.
CONFLICT OF INTEREST
Authors declare no conflicts of interest.
ACKNOWLEDGEMENTS
Authors are thankful to the University authorities and the Head of the Department of Zoology for the facilities.
AUTHORS CONTRIBUTION
The first author, P. Rosina George was engaged with gathering the fish samples and parasites, collection of literature, statistical analysis and composed the original copy while the subsequent author, B. Bharatha Lakshmi was the research supervisor.
ETHICAL APPROVAL
All procedures performed in this study were in accordance with the ethical standards of the University. No specific permissions were required. Samples were collected from Fishing Harbor and different Fish markets in and around Visakhapatnam.
REFERENCES





