South Asian Journal of Life Sciences
Research Article
Comparison the Effect of Fungi (Beauveria bassiana) and Bacteria Bacillus thuringiensis (israelensis) on a Cumulative Mortality of Culex Quinquefasciatus (Diptera: Culicidae)
Aliaa Abdul Aziz Hameed1*, Enas Hatem Kareem Al-Bander2, Fatima Ali Ghanim Al-Obadi3
1College of Science; 2College of Veterinary Medicine; 3College of Education for Pure Science (Ibn Al-Haitham), University of Baghdad, Iraq.
Abstract | Mosquitoes play a serious role on public health transmitting several dangerous diseases such as malaria, filariasis and Encephalitis. So it consider as a vector diseases. Therefor it is important to control its spread using ecology friend’s factors like bacteria Bacillus thuringiensis var. israelensis and fungi Beauveria bassiana. Results revealed that the direct effect of Bacillus thuringiensis on the larvae led to the high mortality (100%) during 3 days while Beauveria bassiana have a cumulative effect on the Mosquito stage lifecycle; 7.3 X 104 more significant effect in the first day but there was no significant in the other days of treatment. In the end period of lifecycle there was no adult emergence and the adult which emergence up normal and die immediately. In conclusion: biological control methods of mosquitoes by using bacteria and fungi are very efficient in eliminating mosquitoes.
Keywords | Beauveria bassiana, Bacillus thuringiensis, Culex quinquefasciatus, Microbial insecticide
Editor | Muhammad Nauman Zahid, Quality Operations Laboratory, University of Veterinary and Animal Sciences, Lahore, Pakistan.
Received | October 22, 2016; Accepted | January 22, 2017; Published | January 29, 2017
*Correspondence | Aliaa Abdul Aziz Hameed, College of Science, University of Baghdad, Iraq; Email: firas_rashad@yahoo.com
Citation | Hameed AAA, Al-Bander EHK, Al-Obadi FAG (2017). Comparison the effect of fungi (Beauveria bassiana) and bacteria Bacillus thuringiensis (israelensis) on a cumulative mortality of culex quinquefasciatus (Diptera: Culicidae). S. Asian J. Life Sci. 5(1): 1-4.
DOI | http://dx.doi.org/10.14737/journal.sajls/2017/5.1.1.4
ISSN | 2311–0589
Copyright © 2017 Hameed et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Chemicals insecticide play an important role in elimination insect that cause several dangerous diseases to human and cancers through the transition to food chain or directly. Although, the chemicals insecticide represents the common approach for the control of the mosquito but the repeated use of chemicals insecticide led to increasing the resistance of insect and increasing the environmental pollution and costs (Allsop et al., 2015). Thus the alternative methods are needed to control noisome insects and limit diseases caused by them. The alternative methods include microbial insecticide (Biological control) which specialized toxic to special species of insect and have no effect on human and other mammals (Rawlins, 1989; Scholte et al., 2004).
Biological control included of bacteria, fungi, viruses, fermons, extract plant (botanical extracts) (McDonald and Lind, 2002) fish, predator like toxorhynchites and protozoa (Meisch et al., 1985; Collins and Blackwell, 2000). The genus culex (Diptera: Culicidae) is one of the most dangerous insect in the nature which play a role in the transmission of pathogens to human. There are two species of this genus: culex pipiens which caused west Nile virus disease and culex quinqiefasciatus which could be transmitted by the nematodes and causing filariasis and Encephalitis (Rawlins, 1989).
Life cycle of culex consist of egg, Larva, pupa and adult stages (Kaddou et al., 2015). Larval stage most infected with pathogens in nature (live in aquatic until adult emergence), adults consider vectors disease because its natural feeding depend on blood of mammals (Helall and Husen, 2013). Beauveria bassiana is a pathogenic and killer to larva and adult mosquito (Bezalwar et al., 2014). So this study was conducted to compare the effectiveness of Beauveria bassiana and Bacillus thuringiensis in controlling the Mosquito.
Materials and Methods
Insect Rearing
The adults of mosquito were collected from Lake of Political Science Collage, University of Baghdad. The diagnosis of the insect as a culex quinquefasciatus was confirmed by the Natural History Museum, Iraq. The adults reared in a box with 20x20x20 cm a rounded with organza cloth. A piece of cotton saturated with 5% sugar solution was placed inter rearing box to feed male of mosquito, while females were fed on the blood of rabbit which linking its parties tightly to prevent movement and putting above the box (Hellal and Husen, 2013). The glasses with 50 ml capacity filled with water inter rearing box to collect egg boats after adult mating then transferred to aquariums with 1 L capacity (used to rear larvae) filled with free chlorine water, fish diet was added constantly as larvae food. The water was replaced every 3 days to keep aquarium clean. Aquariums were reared continually. The experiment performed at the room temperature of 29 ±3 °C.
Treatment of Insect with Bacteria
A total of 15 larvae with 1st instars distributed with 3 replications subjected to Biopesicide ANTROL (Bti.) with concentration of 0.7g/m2 according to the manufacture company. The larvae were monitored every day until they died.
Treatment of Insect with Fungi
Beauveria bassiana SAI were brought from Biotechnology Research Center, General Authority for Plant Protection, Ministry of Agriculture, Iraq. The fungi were refreshed by putting it in the petri dish with PDA media (potato dextrose agar) and chloromingol antibiotic to prevent bacterial growth. The petri dishes incubated in 25 ±2°C and 80 ±5% humidity for 7-10 days (Olivera and Neves, 2004; Dewan, 2010).
Preparation of Fungal Dilution
After the period of fungi incubation, the spores were isolated by harvester (L-shape) and treated with distilled water 5 ml then filtered by a piece of muslin cloth to get stock fungal spores. Three dilutions of fungi were prepared (7.3 x 104, 7.3 x 106, 7.3 x 108) that used to treat 15 larvae with 1st instars distributed with 3 replications. The method of prepare dilution performed according to Kirkland et al. (2004) and Lacy (1997). The number of spores was counted by neubauer counting chamber (haemocytometer) using the following equation (Norris, 1997; Lacey, 1997).
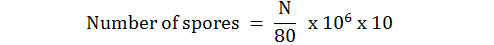
Where;
N is spores collected in 5 haemocytometer chambers, 80 is number of mini boxes in 5 boxes of haemocytometer chamber, 106 is dilution correction factor and 10 is volume correction factor.
Statistical Analysis
Statistical analysis was performed using SAS (Statistical Analysis System - version 9.1). Two-way ANOVA with Interaction and Least significant differences (LSD) post hoc test was used (multiple comparisons), to assess the significant differences among means. P < 0.05 was considered statistically significant (SAS, 2010).
Results and Discussion
Table 1 shows that the differences in the cumulative mortality percentage of mosquito larvae differed significantly due to time of exposure in all treatments. The highest mortality rate was detected in the bacteria treatment as it reached to 100% mortality in the third day. In general the effect of different concentrate of fungi was found significant (P< 0.05) only in the third day. The highest mortality rate (84%) in the fungi treatment was found in the dilution of 7.3x108, while the bacteria treatment has a mortality rate of 100%.
Table 1: The cumulative mortality of mosquito stage treated with fungi Beauveria bassiana and bacteria Bacillus thuringiensis
|
Treatment |
Dilution |
Larvae |
Pupa |
Adult |
||||
|
1 |
2 |
3 |
4 |
5 |
6 |
7 |
||
|
Fungi |
7.3 X 104 |
bc20.0 ± 3.84D |
b42.22± 4.44C |
c66.66± 11.54B |
c73.33± 10.18AB |
bc84.44± 12.37A |
b86.66± 13.33A |
bc86.66 ± 13.33A |
|
7.3 X 106 |
C15.55± 4.44C |
b42.22± 2.22B |
c71.11± 8.89A |
c71.11 ± 8.89A |
c75.55± 11.11A |
b79.99 ± 6.66A |
c79.99 ± 6.66A |
|
|
7.3 X 108 |
C15.55± 2.55C |
b42.22 ± 4.44B |
b84.44± 8.01A |
b86.66 ± 7.69A |
ab91.11± 8.89A |
ab91.11± 8.89A |
ab93.33 ± 6.66A |
|
|
Bacteria |
0.7g/m2 |
a33.33± 6.66C |
a64 ± 11.75B |
a100.00± 0.00A |
a100.00± 0.00A |
a100.00± 0.00A |
a100.00± 0.00A |
a100.00± 0.00A |
|
Control |
- |
d0.00 ± 0.00A |
c2.22 ± 2.22A |
d2.22 ± 2.22A |
d2.22 ± 2.22A |
d2.22 ± 2.22A |
c2.22 ± 2.22A |
d2.22 ± 2.22A |
LSD: 13.22; Means with different small letters in the same column differ significantly (p<0.05), while means with different capital letters in same row differ significantly (p<0.05)
There is no significant difference among the three dilutions (7.3 x 104 , 7.3 x 106 , 7.3 x 108) in the days of pupa stage of 4, 5, 6 days until 7th day when the adult emergence. Depending on Kaddou et al. (2015) who reported that culex life cycle consist of 3 days larva, 3 days pupa and adult emergence in 7th day.
No adult emergence in the end period of life cycle normally and most of it die (100% mortality). The result showed that larva could developed to pupa but not developed to normal adult and died after few minutes or not complete pupa stage as compared with Bacillus thuringiensis (var. i) bacteriopesticide (ANTROL) which considered best than mycopesticide when mortality in larvae treated with ANTROL was 100% during 3 days (period of larva stage) that prohibit the complete life cycle of culex. The different between results could be attributed to the mechanism impact of micro pesticides. Beauveria bassiana effect through osculate when mycelium inter insect body through cuticle and respiratory spiracles, mycelium stimulate to germinate when contact with suitable host (Thomas and Read, 2007).
Fungi kill mosquito Lingeringly (George et al., 2013) so fungicide can spray on rooftops, windows and all rest area that help in reduce number of adult could transfer disease and reduce adult’s ability food (Maehara and Shimazu, 2007). This mechanism impact was seen in Anopheles, culex, Aeds (Scholte et al., 2006; Blanford et al., 2005; Mouatcho, 2010). Fungi infection mosquito larvae reduce the ability of movement and kill 3rd instars in 48 hrs (Bezalwar et al., 2014). Mohantry and Soam (2010) reported that conidia covered all larva body except the head and horns breathing this proved that mycelium inter to haemolymph through cuticle, ventral brush and papillae this consist fungi as keratinophilic. The conidia could stay in water with complete effectiveness for 7 days. The fungi effect on physiological and feeding behaviour of infection mosquito also reduce immunity of mosquito (Weiser, 1991).
Bacillus thuringiensis israelensis (ANTROL) effect through digestive system when spores inter larva body through feeding , so it consider a specific toxic to mosquito larva (Glare and O’Callaghan, 1998). First larva instar is more susceptible to Bti than other instars (Mulla et al., 1990) this depend on mechanism of action of Bti toxin which consist a multistage process, ingestion cry protein by larvae with feeding, solubilisation of cry in midgut which have alkaline medium, proteolytic activation in the insecticidal solubilised protein, apical microvillus membrane of epithelial midgut cell walls have a receptors which bind with toxin, changing in shape allowing toxin insertion into the membrane, toxins doing pores in the cell membrane, because of that osmotic balance disruption and cells swell then burst, thus larva stops feeding. Most larva die in few hours of ingestion (Marrone and Macintosh, 1993; Perez et al., 2005). The result of this search agree with Mario and Montserrat (2012) and Ben-Dov (2014) so it is important to choose the stronger microorganism pathogenic to kill insects (pests) then stopping vector disease with no harmful effect on environment.
Acknowledgments
We would like to thank Assistant Professor Dr. Firas R Al-Samarai for his assistance.
Conflict of Interest
The authors declare that they have no competing interest.
Authors’ Contribution
All authors contributed equally in research and writing of manuscript.
References