Journal of Infection and Molecular Biology
Review Article
Saprolegnia parasitica, A Lethal Oomycete Pathogen: Demands to be Controlled
Showkat Aziz Lone1*, Susan Manohar2
1Department of Zoology and Applied Aquaculture Barkatullah University, Bhopal, M.P India; 2Department of Zoology, Government MGM PG College, Itarsi, M.P India.
Abstract | Fungal infections of fishes with Oomycetes, commonly recognized as water molds, are well-known in fresh water and symbolize the most important fungal group distressing wild and cultured fish. The Saprolegniaceae, in particular members of the genus Saprolegnia, are conscientious for significant infections, involving both living and dead fish and eggs, predominantly in aquaculture amenities. Saprolegniosis, a disease that is characterized by evident white or grey patches of filamentous mycelium on top of the body or fins of fresh water fishes are caused by the members of genus Saprolegnia. Malachite green (an organic dye that is extremely efficient in killing the Saprolegnia pathogen) is used to stay Saprolegnia infection in aquaculture under control. Though the use of malachite green has been barred worldwide because of its carcinogenic as well as toxicological effects and this has resulted in a remarkable re-appearance of Saprolegnia infections in aquaculture. Genus Saprolenia is at the moment economically a very important aquatic fungus, pathogenic to fishes, in particular to catfish, salmon and trout species and necessitate advance study to build up new substitute control strategies. This review focuses on the impact of Saprolegnia parasitica on aquaculture, and different methods used for its control.
Keywords | Fungal infection, Oomycetes, Saprolegniaceae, Saprolegnia, Saprolegniosis
Editor | Tahir Yaqub, University of Veterinary and Animal Sciences, Lahore, Pakistan.
Received | May 31, 2018; Accepted | June 07, 2018; Published | August 22, 2018
*Correspondence | Showkat Aziz Lone, Department of Zoology and Applied Aquaculture Barkatullah University, Bhopal, M.P India; Email: showkataziz964@gmail.com
Citation | Lone SA, Manohar S (2018). Saprolegnia parasitica, a lethal oomycete pathogen: demands to be controlled. J. Inf. Mol. Biol. 6(2): 36-44.
DOI | http://dx.doi.org/10.17582/journal.jimb/2018/6.2.36.44
ISSN (Online) | 2307-5465; ISSN (Print) | 2307-5716
Copyright © 2018 Lone and Manohar. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
The present need to increase the world supply of food for the mounting human population has resulted in the development of exhaustive production of aquatic life under controlled conditions. In intensive fish culture the fishes are reserved at high densities in aninadequate space and provided with supplementary feeding. Hard work have also been in steps forward to culture the fish in sewage-fed ponds or other extraordinary conditions such as to bring up them in warm water used for cooling atomic reactors at optimum temperature all through the year. Rearing the fish in high compactness in limited water with supplementary feeding, keeping it at relatively high temperature level and the risks of pollution are all factors predisposing to the increase of serious disease problems in fishes. Among the freshwater mycotic fish diseases Saprolegniosis disease is one of the most economical diseases. Saprolegniaceous fungi are usually known as water molds, are ubiquitousconstituent of aquatic environment and are capable of growth and reproduction throughout the year. Oomycetes are traditional saprophytic opportunists, multiplying on fish that are physically injured, stressed or infected (Pickering and Willoughby 1982a).Nevertheless there are more than a few reports of Oomycetes as primary infection agents of fish (Willoughby 1978, Pickering and Christie 1980). These water molds are the most significant fungi affecting cultured fish and are measured by some next only to bacterial disease in terms of economic importance to aquaculture (Meyer, 1991). Numerous species of Saprolegniales infecting fish, fish eggs, amphibians and crustaceans have a major impact on freshwater ecosystem (Densmore and Green, 2007; Fernandez-Beneitez et al., 2008; Kiesecker et al., 2001b; McAllister and Robison 2016). Saprolegnia parasitica and Saprolegnia diclina are closely related and are the best studied aquatic oomycetes of the order Saprolegniales (Dieguez-Uribeondo et al., 2007). The Salmon fish farming of countries with temperate climate, such as North-west Euorpe, Chile, Japan and Canada are mostly reported to be infected by S. parasitica and S. diclina. (Hussaein & Hatai, 2002, Gordon Ritchie, personal communication) stated that there are losses estimated at tens of pounds annually in the salmon aquaculture business in Scotland, Scandinavia, Chile, Japan, Canada and USA. 50% “winter kill” in catfish was caused by S.parasitica in USA resulted in economic loss of 25 million (Bruno and Wood ,1999).The rapid increase in the aquaculture production of salmonids has been followed by a rise in several diseases. In particular, Saprolegniosis can account for at least 10% of the annual economic loss in salmonids. (Sandoval-Sierra et al., 2014), also Sandoval-Sierra et al. (2014); represents the first detailed molecular characterization of Saprolegnia species involved in Saprolegniosis in Chile, and the first study showing specific association of different Saprolegnia species with different stages in the salmonid life cycle.
Taxonomy
Early identification: The taxonomic position of S. parasitica and other oomycetes remains uncertain. The taxonomy of oomycetes has been largely based on morphology of reproductive structures and certain biochemical and physiological characteristics; therefore historically there has been a problem in their identification and classification (Dick, 1973; Dick et al., 1984; Beakes et al., 1994a). Saprolegniales are distinguished by mode of zoospore release and their asexual sporangia (Daugherty et al., 1998; Seymour, 1970); oogonia, oospores, and antheridia are used for their identification at species level (Jhonson jr. et al., 2002; Seymour, 1970). Taxonomic confusion regarding isolates of genus Saprolegnia that are pathogenic to freshwater animals has been there as identification to species level is often difficult or even not possible, as many isolates do not build up sexual structures in vitro and therefore information regarding these are inadequate.
Modern identification: Exact species identification is critical for most biological disciplines. The lack of a vigorous classification in the genus, Saprolegnia is principal to the presence of wrongly named isolates in culture collections and of agrowing number of misassigned sequences in DNA database. GeneBank represents the major source of errors for identifying Saprolegnia species as it possesses sequences with misassigned names and also sequencing errors. A recently proposed approach to solve species delimitation (taxonomic diagnostic system) of difficult organisms is the definition of Molecular Operational Taxonomic Units (MOTUs). (Sandoval-Sierra et al., 2014) used 961 sequences of nrDNA ITS from culture collections (461 sequences) and GeneBank (500 sequences), to perform phylogenetic and clustering optimization analysis; as a result identified 29 DNA-based MOTUs in agreement with phylogenetic studies. The resulting molecular clusters supported the validity of 18 species of Saprolegnia and identified 11 potential new ones, also listed a number of incorrectly named isolates in culture collections, misassigned species names to GeneBank sequences, and reference sequences for the species. Study from (Dieguz-Uribeondo et al., 2007) showed that within the S.diclina-S.parasitica complex, six clades could be distinguished. (Beakes et al., 1994) amusingly grouped all Saprolegnia species pathogenic to fish in one group. These analyses recommend that a new method of classification will be required upon conclusion of sequencing projects; such as scheme could possibly result in a number of species being renamed. Transient gene silencing through RNAi is a feasible method to functionally characterize genes in S.parasitica (Saraiva et al., 2014). The analysis of ITS region sequence inconsistency can make possible genus- and species-level recognition of unknown samples from aquaculture amenities and give helpful information on species composition (De la Bastide et al., 2014).
Biology (Life Cycle) of Saprolegnia spp.
Saprolegnia lifecycle is characterized by having asexual production of sporangia and zoospore and sexual reproduction, resulting in the formation of morphologically distinct oospores as that of other species of Oomycetes, as presented in (Figure 1).The mycelia of Saprolegnia are relatively broad, thick hyphe compared to mycelium of Ascomycetes and Basidiomycetes (Dieguez-Uribeondo et al., 2004). The hyphe are mostly aseptate, i.e. coenocytic. Septa are only produced in sexual structures or occasionally in germlings (Dieguez-Uribeondo et al., 2007).
Asexual reproduction: The asexual life stages of S. parasitica are responsible for saprolegniasis (Andersson & Cerenius 2002; Robertson et al.,2009). Asexual reproduction may take place by the production of chlamydospores or gamme, but usually zoospores are produced. Asexual phase is mostly responsible for dispersing free swimming zoospores in order to find new hosts. Zoospores are produced in sporangia, specific factors recognized to set off Saprolegnia zoospore discharge are lack of nutrients (Fuller and Jaworski, 1987) or it may be due to abrupt fall in temperature. In channel catfish (Ictalurus punctatus) a drop of temperature by 100C for 24 hours results in release of elevated number of zoospores (Bly et al., 1992). Amusingly zoospore discharge as a result of cold shock coincides with lowered immune response by the catfish; rising the chance of successful infections by Saprolegnia (Bly et al., 1992). Ribeiro (1983) has also reported release of zoospores as a result of cold shock in some Oomycetes. The asexual spore of Saprolegnia release motile, primary zoospores (Bruno and Wood, 1999; Willoughby, 1994). Primary zoospores are poor swimmers and are active only for few minutes before they encyst, germinate and release a secondary zoospore (Seymour, 1970; Willoughby, 1994). Secondary zoospores are motile for a longer period of time than primary zoospores and are considered the main dispersion and infection spore of Saprolegnia (Willoughby, 1994; Hatai & Hoshiai, 1994). These secondary zoospores have a riniform shape with two lateral flagella. Secondary zoospores of several species of Saprolegnia including S. parasitica, undergo a process, Repeated Zoospore Emergence “(RZE)” or “polyplanetism” in which there is repeated cycles of encystment and zoospore release (Cerenius and Soderhall, 1984a,b; Dieguez-Uribeondo et al.,1994a, 2007). RZE may represent an adaptation to the parasitic mode of life in the Saprolegniales (Beakes et al., 1994; Cerenius and Soderhall, 1985; Dieguez-Uribeondo et al., 2007, 2009).
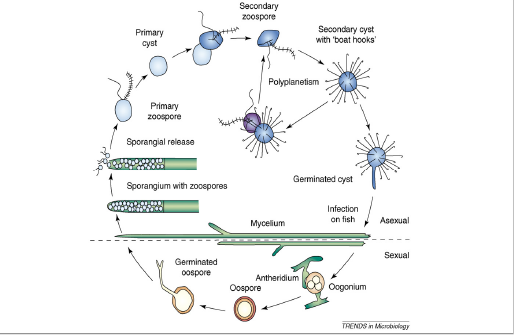
Figure 1: The life cycle of Saprolegnia parasitica. The life cycle consists of both sexual and asexual stages. The figure is not drawn to scale, have been copied from, Andrew J. Phillips.
Sexual reproduction: In saprolegniales sexual reproduction involves the production of antheridium and oogonium gametangia, which unite for fertilization (Pickering and Willoughby, 1982; Seymour, 1970). In most of Oomycetes species male and female sexual reproductive structures appear on a single mycelium (homothallic). In Saprolegnia parasitica fertilization occurs with the fusion of antheridium and oogonium, oospores released are characterized by a thick wall as a protective covering from harsh environmental conditions (e.g. dry or extreme temperature) and enabling them to germinate when conditions are favorable (Beakes and Bartinicki-Garcia, 1989). Oospores produced sexually differ in size, number and shape from one species to other. As there is a variation in oospores, antheridium and oogonia, this makes them only source of morphological variation for species classification and identification (Coker, 1923). The identification of Saprolegnia species has been mostly hindered, as sexual reproduction appears to be difficult to initiate in vivo, and is sometimes impossible in vitro. It is always possible to identify species based on morphological characteristics of their sexual structures (Beakes et al., 1994; Dieguez-Uribeondo et al., 2007).
Diseases Caused by Saprolegnia parasitica
Saprolegniosis, observed in numerous fresh water fishes is characterized mostly by white or greyish cotton-whool like tuft found on infected skin, represented in (Figure 2) and gills of infected adult fish, fry, fingerlings or on eggs,. This disease has received less study than most of the other important diseases of freshwater fish, most likely because fungal infections are usually considered to be the result of an opportunistic, mostly caused by mechanical injury after handling, exposure to extreme pH levels, prolonged exposure to low water temperatures, lack of food, and presence of other microbial infections (Pickering and Willoughby, 1977). In the case of salmonid fish the Saprolegniosis is almost always caused by a single taxon, Saprolegnia parasitica (Willoughby & Roberts. 1992). As skin is mainly affected in case of Saprolegniosis diseases so it is also known as dermatomycosis (Hatai, 1980). The chances of predation of infected fishes are more, as being lethargic due to destruction of epidermis and underlying tissue. There is impaired osmoregulation, respiratory failure and in some cases organ failure of diseased fish in the final stage of infection (Pickering and Willoughby, 1982). There is predation (in the wild) or culling (in aquaculture) in the late stages of Saprolegniosis. It is mostly observed that healthy fishes, with no wounds or parasites are at low risk of being infected by Saprolegnia parasitica.
Eggs of a large variety of freshwater fishes have been reported to be infected by some Saprolegnia species, whose vulnerability is thought to be dependent of egg size. Eggs of common carp (Cyprinus carpio) and catfish (Pelteobagrus fulvidraco) were found to be infected within days as are small in size, in contrast to eggs of salmonid eggs of much larger size, in which infection took much longer to establish (Chukanhom and Hatai, 2004; Cao et al.,2012). At present it is seen that Saprolegnia zoospores cannot infect the salmonid species eggs (Kitancharoen et al., 1997; Thoen et al., 2011). Dead or unfertilized eggs are the places where from infection originates, suggested from reports of fish farmers, so dead eggs serve as a substrate for zoospore colonization, from where mycelia mats engulf and kill nearby eggs (hyphal infection). Hyphal infection is considerd to be devastating in aquaculture hatcheries and can spread quickly between neighbouring eggs (Smith et al., 1985).
Isolation
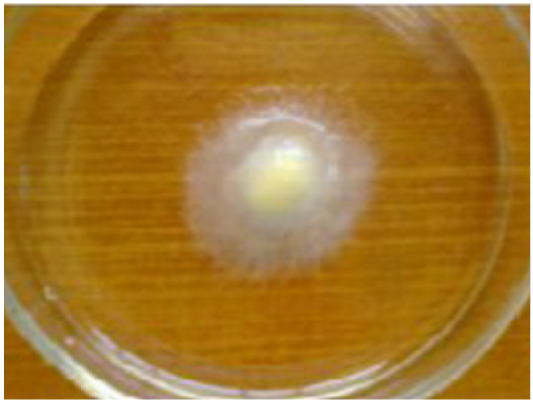
In case of a suspected Saprolegnia infection, isolation of fungi from infected fish is carried out by taking small pieces of infected mussel about 2mm in diameter from different portions of body and thoroughly washed with sterilized distilled water to remove unwanted microorganisms adhered on surface and growing them on selected media (potato dextrose agar Figure 3) and repeatedly transferring growing mycelia tips to new media. Some specific antibiotics, such as streptomycine and streptopenciline are used in the culture medium to exclude the growth of other microbes (bacteria and true fungi). Saprolegnia parasitica can be grown on baits like sterilized hempseeds or rice for the microscopic examination of mycelia, sexual reproductive structures and zoospores (Willoughby and Wood, 1986)(Figure 4). The type of the species isolated is however influenced by type of bait used. The formation of white, cotton-like growth on seeds determines the colonization of zoospores, which are then transferred to selective media plates. Further development in this method was done to enable quantification of zoospore number in water with the use of microstate plates (Thoen et al., 2010).
Identification
Several techniques can be used for the identification of the species isolated. Generic identification of Saprolegniales can be done by microscopic examination of asexual sporangia and mode of zoospore release (Daugherty et al., 1998; Seymour, 1970); species identification is done by examination of sexual structures, oogonia, oospores and antheridia (Jhonson Jr. et al., 2002; Seymour, 1970). Identification to species level is often difficult or even not possible, as many isolates do not build up sexual structures in vitro and therefore information regarding these is inadequate, which has led to the progress of molecular identification methods that are providing new insights into oomycete classification. The use of the Internal Transcribed Spacer (ITS) region, located between the 18S and the 28S rDNA, is commonly used method for identification and includes the 5.8S rDNA sequence. Saprolegnia species (ITS) is approximately 700 bp in length and is considered very suitable for intra-species analysis (Dieguez-Uribeondo et al., 2007). Oomycete species can also be identified by using variable sections from 18S rDNA, 28S rDNA and cox2-gene (Levesque and De Cock, 2004). A recently proposed approach to solve species delimitation (taxonomic diagnostic system) of difficult organisms is the definition of molecular operational taxonomic units (MOTUs). (Sandoval-Sierra et al., 2014) used 961 sequences of nrDNA ITS from culture collections (461 sequences) and GeneBank (500 sequences), to perform phylogenetic and clustering optimization analysis, as a result identified 29 DNA-based MOTUs in agreement with phylogenetic studies. The resulting molecular clusters supported the validity of 18 species of Saprolegnia and identifed 11 potential new ones, also listed a number of incorrectly named isolates in culture collections, misassigned species names to GeneBank sequences, and reference sequences for the species.
Monoclonal antibodies (MAB) (Fregeneda-Grandes et al., 2007a) or SEM (Beakes et al., 1994; Stueland et al., 2005) provided information for the identification of zoospores of strains of S.parasitica. But these techniques are found to be less suitable for the identification of other Saprolegnia species as S. diclina that has much less pronounced cyst morphology (Beakes, 1983). The techniques of highly desirable to better discriminate between various Saprolegnia strains and species, are the development of fingerprinting techniques, such as Multi Locus Sequence Typing (MLST), micro-satellites or one of several polymorphism (Molina et al., 1995; Whisler, 1996).
Control Measures
Fish constitutes cheapest and richest source of protein of good quality, easily digestible. Preventing Saprolegniosis is certainly a near-impossible task, and the use of drugs to both prevent and control the disease has been predictable. The easiest and most lucrative way to control the fungal disease is by good husbandry techniques. Fish health management should focus on to prevent the onset of disease in fish farms Meyer (1991). Minimizing stressful environmental and physiological conditions, maintaining water supply standards and nutritionally complete diets, and isolating infected fish from healthy stocks are some important steps for fungal fish control. Malachite green, an organic drug was used to control Saprolegniosis (Bruno and Wood, 1999; Willoughby and Roberts, 1992). However, it has been banned all over the world, because of its teratogenic properties (Fitzpatrick et al., 1995); Mutagenic properties (Bruno and Wood, 1999). This has resulted in recent resurgence of Saprolegniosis in the aquaculture industry, leading to financial losses world-wide. So it is the demand of the time to find substitute methods to control this mycotic disease. Many alternative control measures for Saprolegniosis have been tested, such as glutaraldehyde; hydrogen peroxide; iodine; the salts potassium permanganate and copper sulphate; and the herbicides diquat, simazine, Three profitable products that contain the chemical formalin, or formaldehyde, have been granted approval from the United States Food and Drug. Formalin is currently used in aquaculture to control Saprolegniosis. However, its use is also likely to be banned in the future. It can control Saprolegnia infection effectively in both fish and their eggs (Marking et al., 1994b; Gieseker et al., 2006); also useful to control Saprolegnia infection in Catfish, but its concentration used must be monitored carefully Bly et al. (1996). High concentrations of sodium chloride (NaCl) can be used for the inhibition of Saprolegnia growth (Marking et al., 1994b; Ali, 2005). Amphotericin B can be used to control infections on channel catfish Bly et al. (1996).. The modified chitosans used for control of Saprolegnia parasitica are regarded as nontoxic Muzzarelli et al. (2001). Bronopol (2-bromo-2-nitropropane-1,3-diol), microbial drug has an efficacy in controlling Saprolegnia infection in rainbow trout and when used at a higher concentration it could also prevent infection in fertilized rainbow trout ova (Pottinger and Day, 1999; Branson 2002). KMnO4 has a defensive role against oxidative stress response; in addition there is a role of FCA in modulating the oxidative stress response and enhancing fish immune response against infections (Zahran and Risha, 2013). Apart from these some other chemicals used for Saprolegniasis control are; copper products (Alderman, 1994; Straus et al., 2009b, 2011), Formaline (Marking et al., 1994; Taylor Francis et al., 1994); UV radiations (J. Heikkinen et al.,2013; Sako, 1985); and Ozone treatment (Sako, 1985). Different concentration of copper sulfate can affect both vegetative and zoosporic stages of water molds, Achlya spp., (Panchai et al., 2016). A concentration of formaldehyde of 500 mg/l or greater, was found to be effective in controlling egg mortality, when administered as disinfection baths, for a period of 15 min, twice a week., without apparent harming the female broods (Benedetto Sicuro, 2016).
Biocontrol of infectious diseases is very important and gaining ground in agriculture (Hofte and Altier, 2010; Qi et al., 2009; Verschuere et al., 2000) and quite a lot of formulations are being used in aquaculture in China but scientific proof is very inadequate. Actinobacteria, of genus Frondihabitans (Microbacteriaceae) effectively inhibit attachment of Saprolegnia to salmon eggs. Low incidence of Saprolegniosis is strongly correlated with a high richness and abundance of this Actinobacteria, so microbial landscapes of fish eggs may provide new sustainable means to alleviate up-and-coming diseases. Live inocula of aquatic Pseudomonas strains, as a replacement for their bioactive compound, can present new (micro) biological and sustainable means to diminish oomycete diseases in aquaculture (Liu Y et al., 2015).Among the fungal community associated with salmon eggs, Trichoderma species may play a role in Saprolegnia suppression in aquaculture (Yiying Liu, 2016). Most recently it has been found that, the complex sterol synthesis pathway for S.parasitica may lead to the identification of some specific targets for inhibition, since sterols are vital for oomycete survival, so there is importance of these molecules as potential drug targets in control strategies (Dahlin et al., 2017). There are few more studies regarding the antifungal effect of probiotics. Lategan et al. (2004) isolated Aeromonas media (strain A199) from eel (Anguilla australis) culture water and were observed to have a strong inhibitory activity against Saprolegnia sp. In a separate study, Pseudomona ssp. M162, Pseudomonas sp. M174 and Janthino bacterium sp. M169 enhanced immunity against Saprolegniasis in rainbow trout. Atira et al., (2012) demonstrated that L. plantarum FNCC 226 exhibited inhibitory activity against Saprolegnia parasitica A3 in catfish (Pangasius hypophthalamus).
Conclusion And Future Research
In recent years, aquaculture has attained a key economic revolution. However infectious diseases of bacterial, viral, mycotic and parasitic origin are the most notable preventive agents in the upgrading of intensified aquaculture. 54.9% bacterial pathogens, 22.6% viruses, 3.1% mycotic agents, and 19.4% parasitic agents are accountable for periodical disease outbreaks in fish cultures (Dhar et al., 2014). S. parasitica represents a demanding problem in the aquaculture development industry (Molina et al. 1995; Van West 2006; Phillips et al., 2008). Saprolegniosis caused by S.parasitica affects aquaculture brood fish and incubating eggs. It is expected that 10% of all hatched salmon succumb to Saprolegniosis, causing major economic loss in an industry accounting for around 30% of the universal fish production for consumption (Molina et al. 1995; Van West 2006; Fregeneda-Grandes et al., 2007; Phillips et al., 2008). The occurrence of Saprolegniosis extends to Asian humid aquaculture systems where more than 80% of fish produced by aquaculture comes from the area (Karunasagar et al. 2003). Given that the international ban of Malachite green in 2002, the need to build up substitute methods for the control of Saprolegniosis has become increasingly urgent. Genomic and proteomic study of S. parasiticaand additional pathogenic oomycetes have provided an exceptional source for studying the molecular processes underlying Saprolegniosis. In addition, the complementary identification of genes and proteins concerned in the immune response of diseased host fish infected through Saprolegniosis will present an understanding of how to put off Saprolegniosis and eventually control the spread of S. parasitica. Future studies of the molecular processes underlying Saprolegniosis in S. parasitica – host fish relations will without a doubt boost our information and understanding of the pathology of S. parasitica, enabling the progress of successful fish vaccines and early recognition of S. parasitica creating a substitute method for controlling Saprolegniosis. Controlling Saprolegniosis is compulsory to make certain sustained development in the aquaculture industry, particularly in Asian steamy aquaculture systems where over 80% of fish produced by aquaculture come from the area. For an industry that accounts for roughly 30% of the worldwide production of fish for consumption, it is vital to prolong studying the fundamental molecular processes of Saprolegniasis in S. parasitica – host fish relations.
AcknowledgementS
The authors are thankful to the HOD of department of zoology and applied aquaculture Barkatullah University Bhopal and also thankful to Dr. Rekha Chauhan for their valuable guidelines to prepare this review paper.
CONFLICT OF INTEREST
The authors have no conflict of Interest.
authors contribution
Showkat Aziz Lone prepared the manscript while Susan Manohar checked the article for any mistakes.
References