Advances in Pharmaceutical and Ethnomedicines
Research Article
Ameliorating Effect of Achillea fragrantissima Against Benzene-Induced Skin Irritation: Histological Study
Mona R Alshathly*1,2, Manal A Alqahtani1
1Biology department, faculty ofscience,King Abdulaziz University, Jeddah,Saudi Arabia; 2Biology department, Northern Border University, Arar054, Saudi Arabia.
Abstract | Organic solvents such as car fuel, which contains benzene and other solvents were known to cause skin irritation and contact dermatitis. Although anti-inflammatory steroids topical medications were used, they may result in side effects in susceptible persons. Herbal preparation was tried in literature. In the present study, Achillea fragrantissima, a wild plant in some areas of Saudi Arabia was used to speed slow-healing wounds. Thus, the study was designed to investigate its efficacy to treat experimentally induced benzene-dermatitis in mice skin. Twenty-five adult mice were shaved and animals were divided into GI as control and GII as benzene painted. The later was further divided into four subgroups; untreated, steroidal medicinal cream treated, a group treated with olive oil extract of Achillea (dried plant soaked in olive oil) and a group treated with olive oil only. Gross and histopathological observation were used to evaluate the treatment effects. Benzene painting produced skin hardening, scaling, epidermal hyperplasia and inflammatory reaction in skin dermis. Decrease in the hypodermal adipose tissue thickness was observed, those pathological changes were much improved or even absent in treated mice. Such results are similar to that observed in the group treated by medicinal cream. Achillea plant could be a source of future effective anti-dermatitis cream medicinal formulation against benzene-induced dermatitis. Olive oil extract of Achillea will be tested and compared to olive oil alone to prove the hypothesis.
Keywords | Skin, Benzene, Dermatitis, Histology, Achillea
Editor | Mayada Ragab Farag, Forensic Medicine and Toxicology Department, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt.
Received | July 24, 2017; Accepted | August 02, 2017; Published | November 04, 2017
*Correspondence | Mona Alshathly, Biology department, faculty ofscience,King Abdulaziz University, Jeddah,Saudi Arabia; Email: mabdulah@kau.edu.sa
Citation | Alshathly MR, Alqahtani MA (2017). Ameliorating effect of achillea fragrantissima against benzene-induced skin irritation: histological study. Adv. Pharm. Ethnomed. 5(1): 1-7.
DOI | http://dx.doi.org/10.17582/journal.ape/2017/5.1.1.7
ISSN | 2310-0575
Copyright © 2017 Alshathly et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
There are different reports investigating the continuous research focus on medicinal plants as one major source of drugs in modern as well as in traditional medicine everywhere in the world. The genus Achillea belongs to the largest family of vascular plants and contains 130 flowering and perennial species (Saeidnia et al., 2011). The name of Achillea is referred to the mythological Greek warrior Achilles, who used Achillea species for healing wounded soldiers during the Trojan War (Cheers, 1999) also known as “Qaisoom” in Arabic.
Given the widespread nature of this genus, Achillea species are used as medicinal plants against diarrhea, flatulence, emmenagogue, wound healing purposes (Honda et al., 1996) fever, common cold and digestive complaints (Si et al., 2006; Akkol et al., 2011). The herb has a strong smell and a bitter taste, for this reason it is usually mixed with sugar when used orally. It has also anti-inflammatory effects when used topically (Lemmens-Gruber et al., 2006; Zhang et al., 2014). Furthermore, the species Achillea millefolium could be beneficial in preventing and/or treating neurodegenerative diseases like Alzheimer and Parkinson (Elmann et al., 2011) as well as in 65 cosmetic formulations (Schmidt et al., 2014) while Achillea eriophora and Achillea biebersteinii have shown to induce protective properties against oxidation, cytotoxicity and DNA damage (Varasteh-Kojourian et al., 2017).
The flora of Saudi Arabia represents one of the richest areas in the Arabian Peninsula and comprises a very important genetic resource of crop and medicinal plants (Shahat et al., 2013). Furthermore, Achillea fragrantissima is found as a wild plant in some areas (Elsharkawy et al., 2014).
It is a well-known fact that many skin impairments, especially those that require the treatment with anti-inflammatory agents and substances that are able to protect the skin from water loss after such impairment, will be managed and treated by applying the plants and their extract topically (Houghton et al., 2005).
Benzene is used as a chemical intermediate for the production of many important industrial compounds, such as synthetic rubber, phenol, nylon, dyes and detergents (Fruscella, 2000). On the other hand, it is known to be cytotoxic through both systemic and local side effects (Krewski et al., 2000). Jia et al. (2002) reported that benzene could induce dermatitis in most exposed workers. Experimental work carried out by Lyu et al. (2013) on mice model of contact dermatitis proved that benzene derivative1-fluoro-2,4-dinitrofluorobenzene (DNFB) results in inflammatory effects on painted skin.
Skin irritation is a complex biological event, commonly described by the clinical response edema, dryness and/or redness (erythema) to chemical or physical stimulus resulting in inflammation at the site of contact after a single or multiple occurrences. Medical therapy of such event includes topical treatment with anti-inflammatory creams, lotions as well as topical corticosteroids (Carroll and Fleischer, 2004; Wat and Dytoc, 2014).
Research Problem
Organic solvents such as car fuel, which contains benzene and other solvents were known to cause skin irritation and contact dermatitis. Although anti-inflammatory steroids topical medications are used as treatment, they may result in side effects in susceptible persons. Therefore, herbal medication will be explored as alternative or complementary treatment.
Research Objectives
In this study, the main aim is focused on evaluating the possible ameliorating effect of topical application of olive oil extract (known for its common and traditional application as extractant) of Achillea fragrantissima against benzene-induced dermatitis in mice model in order to give scientific justification for its usage in treatment of inflammatory skin conditions. Histological studies is also applied to evaluate the success of such new herbal medication compared to the well-known medicinal steroid topical cream.
RESULTS
Gross Morphology
Gross morphology of dorsal back skin of mice from control group was shown in (Figure 1). After two weeks of daily painting with benzene, patchy hair-loss and redness (erythema) were observed (Figure 1B). Skin dryness and scaling were demonstrated after three weeks (Figure 1C) where in those animals the skin was adherent to underlying muscle and bled extensively upon removal. Application of Achillea in olive oil markedly decreased hair loss, dryness and scaling (Figure 1D). Similar improvements were obtained using olive oil alone (Figure 1E) and medicinal cream Metazone (Figure 1F) However, the best response occurred with olive oil extract of Achillea.
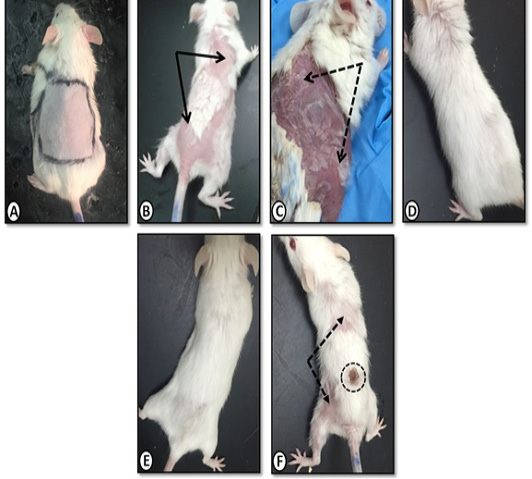
Figure 1: Gross morphology of mice dorsal back skin of A. Control group with normal skin appearance. B. Benzene painted (two weeks) showing patchy hair loss (arrows)and redness (erythema). C. Benzene painted (three weeks) showing skin dryness and scaling (dotted arrows). D. Achillea in olive oil treated skin showing normal skin with no dryness or scales E. Olive oil treated skin with similar improvement. F. Medicinal cream (Metazone) treated showing moderate improvement, still scaling (dotted arrow) and hair loss. Dotted circle (site of skin biopsy).
Histological study
Histological Structure of Control Mice Dorsal Skin:
Dorsal back skin of control mice consisted of the well-known layers of mammalian skin, namely superficial epidermis, deep dermis and the deepest hypodermis. The epidermis is relatively thin, consisted of four to five cell layers with very thin keratin layer. The nuclei of keratin ocytes are vesicular and the cytoplasm was acidophilic (Figure 2A) and (Figure 3A).
Dermis consisted of collagen fibers randomly distributed among hair follicles and their sebaceous glands and clearly demonstrated in sections stained with Masson’s trichrome stain (Figure 4A).
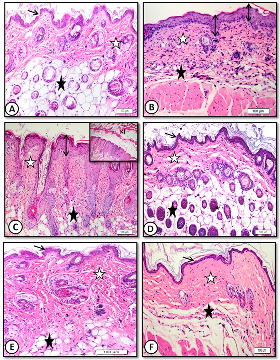
Figure 2: Low magnification (x20) of mice dorsal back skin stained with H&E showing A. control with normal epidermal thickness (thin arrows), dermis (white star) and hypodermis (black star). B .benzene painted (two weeks) increased epidermal thickness (arrows) and dermal inflammatory cells with decreased adipose tissue (black star). C. benzene painted (two weeks) marked increase in epidermal thickness and keratin layer (KL) with desquamation. Note ridge elongation (double head arrows). D. Achillea in oil treated skin showing normal epidermal thickness (arrows) and dermis (white star) with normal hair follicles and sebaceous glands (black stars). E. Oliveoil treated skin with similar improvement. F. medicinal cream treated skin with normal epidermal thickness(arrow), few hair follicles and inflammatory cells (stars).
Histological Structure of Benzene Painted Skin:
Repeated daily painting of mice dorsal back skin with car fuel (benzene) for two weeks resulted in an increase of epidermal as well as dermal thickness. Decreased in thickness of adipose tissue layer was also observed after two weeks (Figure 2B) and (Figure 3B).

Figure 3: High magnification (x60) of mice dorsal back skin stained with H&E showing: A. control with normal epidermal thickness (double heads arrow). B. Benzene painted skin (three weeks) showing increase in epidermal thickness (double head arrow),dermis (white star) is infiltrated with mononuclear and mast cells (dotted arrows). C. Benzene painted skin (three weeks) showing marked increase in epidermal thickness and ridge elongation (double head arrows). D. Achillea in oil treated showing normal epidermal thickness, hair follicles and sebaceous glands (white star) with absence of inflammatory cells (black star). E. Olive oil treated skin showing normal epidermal thickness (double head arrows), few sebaceous glands (white star) and inflammatory cells (black star). F. Medicinal cream treated skin with normal epidermal thickness (double head arrow), few hair follicles and inflammatory cells (stars).
The dermis showed increase in inflammatory cells especially mast cells (Figure 3B). After three weeks, marked increase in epidermal thickness with elongation of epidermal ridges and a decrease in adipose tissue were observed (Figure 2C) and (Figure 3C).
Histological Structure of Dorsal Skin of Treated Groups:
Achillea in Olive Oil
Topical treatment of benzene painted skin with Achilleain olive oil for one week resulted in marked restoration of normal skin histological structure. Skin showed normal thickness of both epidermis and dermis with decreased in inflammatory and mast cell infiltration. Hair follicles appeared to be re-grown (Figure 2D) and (Figure 3D).
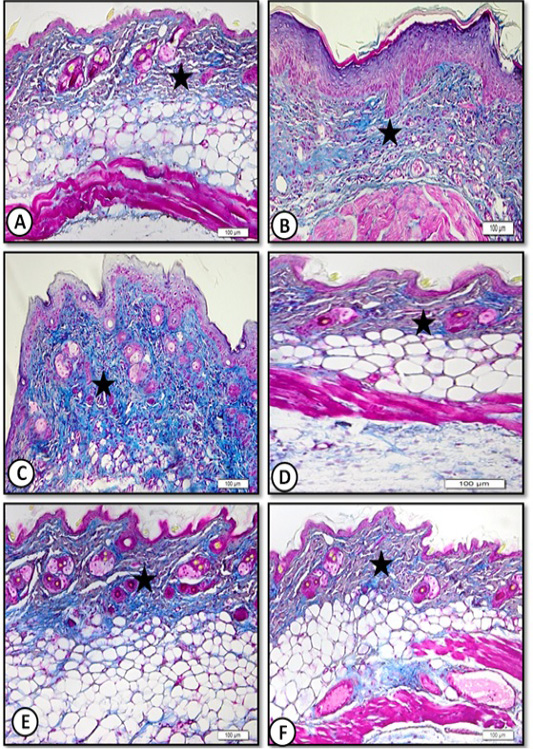
Figure 4: Low magnification (x20) of mice dorsal back skin stained by Masson trichrome for collagen fibers showing A. Control with normal collagen content (star). B. Benzene painted with increase collagen(star). C. Benzene painted (three weeks) with increase collagen content (star). D. Achillea in oil treated showing normal collagen content (star). E. Olive oil treated skin showing normal collagen content (star). F. Medicinal cream treated skin with normal collagen content (star).
Olive Oil
Similar improvements were observed in samples treatedwith olive oil alone. There was decrease in epidermal thickness. Dermal inflammatory cells markedly decreased. Few sebaceous glands were observed (Figure 2E) and (Figure 3E).
Medicinal Cream
Medicinal cream also restored normal epidermal thickness but still dermis showed less hair follicles and sebaceous glands (Figure 2F) and (Figure 3F).
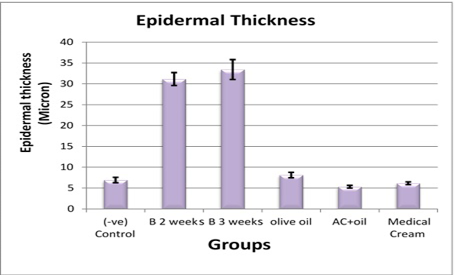
Morphometric Study
Measurements of epidermal thickness by pro-image analyzer provided statistical confirmation of the previous histological observation (Table 1 and Figure 5).
DISCUSSION
The anti-inflammatory properties of Achillea fragrantissima olive oil extract prepared by traditional methods for topical administration have not been investigated enough although other methods of extraction showed promising results.
The chemical composition of this genus in relation to its therapeutic uses was studied by different authors. Vitalini et al. (2011) reported that Achillea species are rich in many bioactive compounds with potent anti-oxidant activities such as flavanol glycosides. Those active components in olive oil extract has potent free radical scavenging activity and was reported to have ability to inhibit lipid peroxidation. Akram (2013) provided mini-review on Achillea millefolium and reported that it is considered an important medicinal plant with various pharmaceutical applications including skin diseases such as bruises, boils, psoriasis and eczema.
Schultz et al. (2001) and Dias et al. (2013) studied the chemical composition of wild and commercial Achillea millefolium and found that they are rich in many natural antioxidant compounds for treatment or prevention of related diseases including flavonoids, tocopherols, phenolic acids, monoterpenes, sesquiterpenes and caffeoylquinic acid derivatives. It is also worth mentioning that plants with high phenol and flavonoid contents seems to show direct proportion to high antioxidant activity (Varasteh-Kojourian et al., 2017). These findings concur with the importance of studying the Achillea plants for their properties. Differences in measured content of Achillea species from various studies may be mainly due to diversities of the analytical methods, extraction methods, variety, developmental phase and geographical origin of the plant. Alas, most studies have shown them all containing similar components that perform anti-inflammatory and other therapeutic and protective properties.
Table 1: Epidermal thickness of back skin control mice and treated groups
|
Group |
(-v) Control |
Two weeks Benzene |
Three weeks Benzene |
Olive oil |
AC + oil |
Med. Cream |
|
Mean ± Std. Dev. |
6.933 ± 1.584 |
31.164 ± 3.789 |
33.439 ± 5.806 |
8.118 ± 1.579 |
5.327 ± 0.829 |
6.166 ± 0.829 |
|
P-value |
1.23E-14* 1.15E-15# |
4.51E-14* 3.87E-15# |
2.27E-15* 2.37E-16# |
5.43E-15* 5.36E-16# |
P-value with significant differences between the groups ≤ 0.05.
Notes: * Highly significant differences between benzene painted two weeks and all groups. # Very highly significant differences between benzene painted three weeks and all groups (P≤ 0.05). AC, Achillea, Med., medicinal, Std. Dev., standard deviation.
In this study, painting of mice dorsal skin with benzene was found to produce pathological changes in the form of early hyperemia and hair loss after two weeks of treatment and progressed to skin hardening, scaling and adherence to underlying tissues after the third week. Histological studies showed marked increase in both epidermal and dermal thickness, inflammatory and mast cell infiltrate in dermal tissue. Marked decrease in hair follicles and hypodermis adipose tissue were also evident. Similar changes were reported in guinea pig skin painted by benzene (Al-Saggaf et al., 2011).
It is accepted that chemical irritants may cause skin irritation via two distinct pathways; impairment of the barrier function of the stratum corneum and/or by direct effects on the cells of the keratin ocytes of the epidermal layer (Kartono and Maibach, 2006; Welss et al., 2004; Wilhelm et al., 1993).
Chatterjee et al. (2005) found that application of benzene to hairless rat skin result in skin irritation, and increase inflammatory cytokines released from inflammatory cells. Lyu et al. (2013) found that repeated skin painting with DNFB in mice produced histopathological changes specific for contact dermatitis including epidermal hyperplasia, spongiotic changes and mononuclear cell infiltration in the dermis, which are similar to the present findings.
When Achillea fragrantissima extract in olive oil was used as treatment for the benzene-induced irritation, reversed histological changes were observed. A marked restoration of normal thickness of both epidermis and dermis that was quiet equally to the control group. In addition, a noticeable decreased in inflammatory and mast cell infiltration and mature hair follicles appeared to be re-grown.
Fibroblasts play an important function in proliferation (Jongkind and Verkerk, 1984; Mastromonaco et al., 2006) and can be accounted for many applications in tissue engineering, genetics and cell reprogramming (Wong et al., 2007). Following sever damage, they infiltrate wounds and secrete growth factors, cytokines and fibrotic extracellular matrix factors such as collagen I and fibronectin (Velnar et al., 2009) in order to initiate and commence healing.
Given the presence of mature hair follicles in epidermis
layer of the skin as well as fibroblasts and collagen in the dermis layer is the proof of completion of healing (Akkol et al. 2011), these observations indicate a commenced healing process that may disclose the anti-inflammatory properties of the used extract.
Furthermore, a study using Achillea millefolium oil extract resulted in improvement of skin hydration of irritant human skin induced by application of 8% sodium lauryl sulfate after three days of treatment (Tadić et al., 2017). Another study on the anti-inflammatory activity of Achillea fragrantissima flower extract showed reduction of carrageenan-induced rat paw edema by 48%, which was nearly equally effective to that produced by the standard Diclosal Emulgel medication (47% reduction of edema), indicating an anti-inflammatory effect (Maswadeh et al., 2006). In consideration with the present results and previously published ones, it is imminent to suggest the strong anti-inflammatory properties of Achillea as a topical treatment of skin irritation.
It is noteworthy to state that the results from medicinal treatment (Metazone) group used although showed improved skin condition was not as prominent as those of the Achillea extract in olive oil group were.
Conditions and recommendations
Taking into account traditional usage of Achillea in skin inflammations treatment, the assessment of its ameliorating and anti-inflammatory properties was achieved in this study.
Achillea could be a source of future effective anti-dermatitis medicinal cream formulation against benzene-induced dermatitis. The mechanism by which Achillea provide protection is going on in the same work using both immunohisto chemically and biochemical assay of inflammatory mediators and total antioxidants.
MATERIALS AND METHODS
Preparation of Achillea for Topical Application
Achillea fragrantissima plant (leaves and stems) was collected from Arar region, Saudi Arabia in February 2015 and dried under shade. Dried plant was then immersed in olive oil for one month in ration of (1:5), filtered and stored in dark sterile bottles until further use.
Animals
Female mice (N= 25) weighing 40gm were obtained from animal house of the King Fahd Medical Research Center (KFMRC) in Jeddah, Saudi Arabia. The animals were acclimatized to laboratory conditions for seven days before carrying out the experiment. Animals were housed in plastic cages in an air-conditioned room at 22 ± 10 C and allowed free access to standard animal chow and water.
Induction of Skin Contact Dermatitis
For carrying out the experiment, animals were anesthetized with ether. Hair on the dorsal surface of the mice was removed carefully with an electrical shaving machine, taking care not to damage the stratum corneum (the outermost layer of the epidermis). The sites of painting were marked as square areas (3x2 cm) on the shaved surfaces (Figure 1A).
Animals were grouped as follows:
The control group (GI): (n=5) where marked skin region was painted by distilled water.
The experimental (GII): (n=20) where shaved skin was painted by car fuel (benzene) four times daily every two hours using special paint brush soaked in 1 ml of benzene (Chatterjee et al., 2005).
Pained skin regions were observed and photographed for signs of skin irritation for two weeks. Animals were subdivided into four subgroups:
Subgroup GII A: (n=5) as benzene untreated group.
Subgroup GII B: (n=5) as animals were painted twice daily (five hours apart) by previously prepared olive oil extract of Achillea.
Subgroup GII C: (n=5) as animals were painted twice daily (five hours apart) by olive oil only.
Subgroup GII D: (n=5) as animals were treated by the medicinal cream (Metazone).
After three weeks treatment, punch biopsies (2x2 mm) were taken from both control and experimental animal groups. Skin samples were fixed in 10% neutral buffered formalin, processed routinely for paraffin embedding in King Abdul aziz University pathology lab. Five-micron thick sections were stained by Haematoxylin and Eosin (H&E) for general structure and Masson trichrome for collagen fibers. The stained sections from all groups were examined by OLYMPUS DP 72 light microscope connected to digital camera provided by pro-image analyzer program version 2 and compared for histopathological changes.
Acknowledgements
We would like to extend our thanks to Prof. Soad Shaker Ali, anatomy department, Faculty of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia for her dedicated guidance and comments throughout this project.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
Authors Contribution
Mona Alshathly and Manal Alqahtani worked on the concept and objectives of the project. Manal Alqahtani did the experimental load and data analysis. Mona Alshathly and Manal Alqahtani worked in writing and formatting the final manuscript.
REFERENCES