Research Journal for Veterinary Practitioners
Case Report
Septicemic Salmonellosis in Pre Weaned Calves Caused by Salmonella dublin
José Azael Zambrano Uribe1, Fernanda Morcatti Coura2, Philipe Pimenta Nunes1, Marcus Vinícius Prado Silva2, Antônio Último de Carvalho1, Matheus Vilardo Lóes Moreira1, Filipe Lucas de Melo Mendonça1, Lauranne Alves Salvato1, Rodrigo Melo Meneses1, Roberto Maurício Carvalho Guedes1, Andrey Pereira Lage2, Marcos Bryan Heinemann3*, Elias Jorge Facury Filho1
1Departamento de Clínica e Cirurgia Veterinárias, Escola de Veterinária, Universidade Federal de Minas Gerais, Av. Antônio Carlos, 6627, CEP31270-010, Belo Horizonte, Minas Gerais, Brazil; 2Departamento de Medicina Veterinária Preventiva, Escola de Veterinária, Universidade Federal de Minas Gerais, Av. Antônio Carlos, 6627, CEP31270-010, Belo Horizonte, Minas Gerais, Brazil; 3Departamento de Medicina Veterinária Preventiva e Saúde Animal, Faculdade de Medicina Veterinária e Zootecnia, Universidade de São Paulo, Av. Prof. Orlando Marques de Paiva, 87, CEP 05508-270, São Paulo, São Paulo, Brazil.
Abstract | This report describes clinical, macro and microscopic changes observed in an outbreak of septicemic salmonellosis in pre-weaned calves caused by Salmonella. The necropsy of calves was performed and gross lesions were identified and characterized. The differential diagnosis included hemoparasites (Anaplasma, Babesia bigemina, Babesia bovis and Ehrlichia spp.), Leptospira and Escherichia coli. The course of infection was three days from the moment calves got sick until death. Some of the clinical signs included apathy, reduced solid food ingestion, hyperthermia and mucosal congestion. Postmortem findings were mostly congestion of oral, ocular and rectal mucosa; petechiae were observed in the vaginal, bladder and sublingual mucosa, serosa of the rumen, reticulum, omasum and small intestine; the lungs were congested. Microscopic examination of the lung was characterized by inflammatory infiltration mostly of neutrophils, fibrin and moderate multifocal haemorrhagic alveolar and bronchial lumen. Vasculitis, necrosis and thrombosis in the liver, gallbladder, spleen, and hypophysis were also observed. Salmonellosis is an important disease of dairy cattle resulting in increased morbidity and mortality of affected animals. This report offers a better understanding of the clinical and pathological characteristics of the disease and demonstrates the importance of diagnostic approaches in cases of Salmonella dublin infections.
Keywords | Salmonella dublin, Cattle, Diagnosis, Systemic salmonellosis, Clinical pathology
Editor | Muhammad Abubakar, National Veterinary Laboratories, Islamabad, Pakistan.
Received | August 17, 2015; Revised | September 14, 2015; Accepted | September 16, 2015; Published | October 11, 2015
*Correspondence | Marcos Bryan Heinemann, Universidade de São Paulo, Av. Prof. Orlando Marques de Paiva, São Paulo, Brazil; Email: marcosbryan@usp.br
Citation | Uribe JAZ, Coura FM, Nunes P, Silva MVP, de Carvalho AÚ, Moreira MVL, Mendonça FLM, Salvato LA, Meneses RM, Guedes RMC, Lage AP, Heinemann MB, Filho EJF (2015). Septicemic salmonellosis in pre weaned calves caused by Salmonella dublin. Res. J. Vet. Pract. 3(3): 69-75.
DOI | http://dx.doi.org/10.14737/journal.rjvp/2015/3.3.69.75
ISSN | 2308-2798
Copyright >© 2015 Uribe et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Salmonellosis is an important disease of dairy cattle resulting in increased morbidity and mortality of affected animals as well as a high cost of treatment. The serovars typhimurium and dublin are the most common serovars associated with disease outbreaks. S. typhimurium is commonly isolated in cases of enteritis in calves less than 2 months old, while S. dublin is often present in cases of septicemia, meningoencephalitis and septic arthritis—associated or not with enteritis (Mohler and House, 2009; Costa et al., 2012). Furthermore, S. dublin has been isolated in cases of pneumonia and dry gangrene of the extremities in calves (Mee, 1995).
There are few case reports in the literature describing the clinical and pathological findings of calves naturally infected with S. dublin (Marques et al., 2013). This data could contribute to a better understand of disease pathogenesis and help clinicians to diagnose, control and prevent infections. Moreover, a report on an outbreak of S. dublin in a dairy farm characterized by abortion highlighted the zoonotic importance of this serovar (Mateus et al., 2008). Due to the importance of salmonellosis and the lack of case reports in the literature, we describe here the clinical, macro- and microscopic findings of salmonellosis outbreak infection in 60- and 90-day-old calves at a dairy farm in Minas Gerais, Brazil.
Clinical cases occurred in December 2013 and affected eight pre-weaned calves between 60 to 90 days old. We performed a clinical examination of the eight affected animals (Dirksen et al., 1993). The parameters also evaluated included rectal temperature (RT), packed-cell volume (PCV), rickettsemia caused by Anaplasma marginale and parasitemia caused by Babesia spp.—these were all done with a blood smear.
Blood samples with anticoagulant tripotassium ethylenediaminetetraacetic acid (K3EDTA) were collected from two animals (calves 3 and 4) with clinical signs of fever and tachypnea. Because of the distance of the Veterinary Hospital and the Dairy Farm and the clinical conditions of the animals, it was possible to collect only one blood sample from calf 4 on the first day of clinical manifestation, while calf 3 had blood samples taken during the course of clinical signs to total three blood samples. Moreover, two blood samples without anticoagulant were collected from calf 3. This corresponded to the first two days of clinical manifestation. On these samples, we measured alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma glutamyl transferase (GGT), alkaline phosphatase (ALP), glucose, total protein, albumin, urea and creatinine.
Necropsy was performed immediately after the death of the four calves (calves 1, 2, 3 and 4). Fragments of lung, spleen, pituitary, liver, kidney, abomasum, intestine and mesenteric lymph nodes were collected in two aliquots—one was fixed in 10% formaldehyde buffered solution for histological examination, and the other aliquot was sent for bacteriological examination. For hemoparasites (Anaplasma, Babesia bigemina, Babesia bovis, Ehrlichia spp.) diagnosis, blood samples were collected from the tip of the tail for blood smears and Romanowsky staining.
Kidney and lung fragments were used to diagnose Leptospira. The growth of Leptospira was observed using dark-filed microscopy for 28 days (Turner, 1970). The serological diagnosis of leptospirosis was done using the Microscopic Agglutination Test (MAT) (Galton et al., 1965) with a panel of reference Leptospira serovars.
Escherichia coli and Salmonella spp. were identified according to Quinn et al. (1994) and Waltman (2000). Colonies suggestive of Salmonella spp. were subjected to biochemical tests and conventional PCR (Amavisit et al., 2001) to confirm the genus Salmonella. Then, the disk diffusion antimicrobial susceptibility test was performed according to the CLSI (CLSI, 2014). The characterization of the serovar was performed at the Reference Laboratory at the Instituto Oswaldo Cruz/Fundação Oswaldo Cruz (Rio de Janeiro, RJ, Brazil).
Calves were negative for hemoparasites and Leptospira spp. Salmonella spp. was isolated in the lung and liver fragments as well as the bile samples from the four calves that were necropsied. The S. dublin serovar was identified in the tissues described above in all affected animals. The results of in vitro antimicrobial susceptibility test showed that Salmonella isolates were penicillin resistant, had low sensitivity to enrofloxacin and streptomycin, and were sensitive to cephalothin, amoxicillin, gentamicin and florfenicol.
Prior to establishing the etiologic diagnosis of salmonellosis, four calves that got sick died. After diagnosis, four more animals got sick and were treated with florfenicol. Flunixin meglumine was also administered intramuscularly in these animals in addition to hydration and omeprazole orally over five days. After the treatment, no more clinical cases were observed.
The clinical signs observed in the eight calves were similar varying only in severity. The course of infection was three days from the moment calves got sick until death. On the first day of infection apathy, we noted reduced solid food ingestion with consistent milk ingestion, rectal temperature of 41°C to 42°C, yellow ocular and vaginal mucous membranes and retraction of the eyeball.
On the second day of infection there was intense apathy and anorexia as well as an absence of rumen movements. In the sublingual and vaginal mucosal areas there were several petechiae. Tachycardia, tachypnea were also detected. On the third day of infection the clinical signs described above continued in addition to abduction of the thoracic limb, panting and tongue protrusion. Necropsy was performed soon after the calves died (calves 1 and 2) or were seriously ill and were euthanized (calves 3 and 4).
Animal 3 had hypoproteinemia, associated with a progressive increase in plasma fibrinogen. The gradual increase in the packed-cell volume, the erythrocyte counts and hemoglobin content were suggestive of hemoconcentration. Moreover, during the third exam, the calf presented an intense elevated myeloid lineage precursors associated with monocytosis, although the global leukocyte count was within reference values. This pattern was also observed in white cell blood counts from previous days but with less
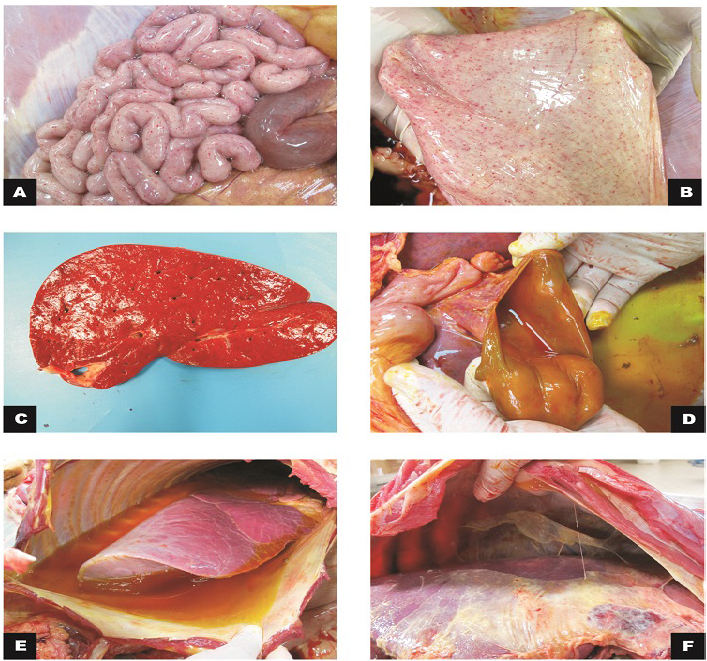
Figure 1: Macroscopic findings in calves
A) Small intestine, jejunum: serosa with marked diffuse petechiae (calf 1); B) Bladder: mucous membrane with marked diffuse petechiae (calf 1); C) Liver: slight increase in volume and orange colour (calf 2); D) Gallbladder: moderate thickening of the wall (calf 2); E) Thoracic cavity and lungs: moderate hydrothorax associated with petechiae and discrete multifocal ecchymosis in the parietal pleura; F)Cranial, middle and right caudal lung lobes not collapsed with reddish cranio-ventral portions (calf 3).
intensity. On the second day, there was neutropenia. Animal 4 had mild anemia, a slight increase in plasma fibrinogen, intense left shift and mild monocytosis. In the first serum biochemistry analysis of calf 3, the ALT, AST and GGT values were mildly elevated and the total serum protein was moderately reduced similar to albumin, globulins and glucose. These findings were also observed in the second exam.
During the external inspection portion of the necropsy we noted congestion of the oral, ocular and rectal mucosa as well as petechiae in the vaginal and sublingual mucosa. Subcutaneous edema and ecchymosis on the back muscles were observed. There was a great amount of gelatinous and yellowish peritoneal fluid in the peritoneal cavity that also involved the periphery of the duodenum in calf 3. Petechiae were observed in the serosa of the rumen, reticulum, omasum, small intestine and bladder mucosa. The mesenteric lymph nodes were elongated and hemorragic, and the mesenteric vessels were ectasic. The liver was enlarged with a yellow-orange color. In the thoracic cavity, there was abundant gelatinous yellow liquid in half of the cavity (Figure 1). Petechiae were observed in the visceral and parietal pleura. The lungs were congested with diffuse red
Table 1: The gross findings of the four calves necropsied
|
Gross findings |
Calves |
|
Congestion of the external mucosa |
1, 2, 3 and 4 |
|
Subcutaneous edema and ecchymosis on the back muscles |
1, 2, 3 and 4 |
|
Gelatinous and yellowish peritoneal fluid in the peritoneal cavity that also involved the periphery of the duodenum |
3 |
|
Petechiae in the serosa of the rumen, reticulum, omasum, small intetine and bladder mucosa |
1, 2, 3 and 4 |
|
Mesenteric lymph node were enlongated and hemorragic, and mesenteric vessels are ectasic |
1, 2, 3 and 4 |
|
Liver enlarged with a yellow-orange color |
1, 2, 3 and 4 |
|
Toracic cavity with hydrothorax |
1, 2, 3 and 4 |
|
Petechiae in visceral and parietal pleura |
1, 2, 3 and 4 |
|
Lungs congested with diffuse red consolidation and emphysema in the final portion of the caudal lobes |
1, 2, 3 and 4 |
|
Leptomeninge congested |
1, 2, 3 and 4 |
|
Abdominal cavity, liver and diafragma with severe fibrinous adhesion between the visceral peritoneum of the liver and diaphragm |
4 |
|
Enlarged omentum hemorrhage |
4 |
|
Serous and abomasal mucous membrane had necrotic foci and fibrin |
4 |
consolidation and emphysema in the final portion of the caudal lobes. The leptomeninge was also congested.
During the necropsy of calf 4, the abdominal cavity, liver and diaphragm had severe fibrinous adhesion between the visceral peritoneum of the liver and the diaphragm. Fibrin diffusely distributed throughout the abdominal cavity was observed (Figure 1). Diffuse hemorrhage with approximately 10 cm in diameter was evidenced in the omentum. The serous and abomasal mucous membrane had necrotic foci and fibrin. Table 1 summarizes the gross findings.
The microscopic findings are summarized in Table 2. Microscopic examination of the lung of the four calves showed inflammatory infiltration mostly of neutrophils, amorphous proteinaceous material (edema), fibrin and moderate multifocal hemorrhages in the alveolar and bronchial lumen. In the alveolar septa, there was lymphocytic inflammatory infiltrate associated with macrophages and neutrophils and intense multifocal lymphocytic vasculitis with thrombosis in the capillaries (Figure 2). Therefore, we diagnosed the subject with broncho-interstitial neutrophilic and lymphohistiocytic-associated pneumonia with thrombosis and lymphocytic vasculitis. There was also vasculitis, necrosis and thrombosis in the liver, gallbladder, spleen and hypophysis (Figure 2).
The large intestine of one of the calves had lost the continuity of the epithelium overlayer and of the superficial lamina propria. There was exposure of the submucosa associated with neutrophilic inflammatory infiltrate, fibrin and intense multifocal coalescing of the thrombosis. This was compatible with a fibrinonecrotic and neutrophilic colitis associated with thrombosis (Figure 2).
The clinical changes seen in the affected calves, such as fever, lethargy, lack of appetite and jaundice, agree with the clinical signs observed in an outbreak of S. dublin (Marques et al., 2013). The severity of the clinical signs and course of the salmonellosis in cattle infected with S. dublin depend on factors such as infectious dose, route of infection, host resistance and strain, which often result in severe clinical manifestations in affected animals (Nielsen, 2013).
The hemoconcentration observed in the blood counts of calf 3 is a consequence of progressive dehydration. However, the total protein values did not follow the increase in hematocrit and the concentration of red blood cells as would be expected in cases of dehydration. Similar results were observed by Santos et al. (2002).
Despite the progressive increase in fibrinogen, the total protein showed a moderate reduction associated with a reduction in albumin and globulin values. Fibrinogen in cattle increases acute inflammation (positive inflammatory acute phase protein) while the albumin decreases (negative inflammatory acute phase protein) (Meyer and Harvey, 1998). Furthermore, the globulins tend to increase as the inflammation becomes chronic—this is not observed in the testing because the course was acute.
In serial evaluation of white blood cell counts of calf 3, we found that bone marrow precursor cells of the myeloid lineage associated with monocytosis. These findings differ from those reported by Marques et al. (2013). The changes observed in this present report are probably because the body tried to overcome S. dublin infection and stimulated production and release of granulocytes and monocytes. Neutropenia occurs due to the intense mobilization of blood neutrophils to the various sites of S. dublin infection
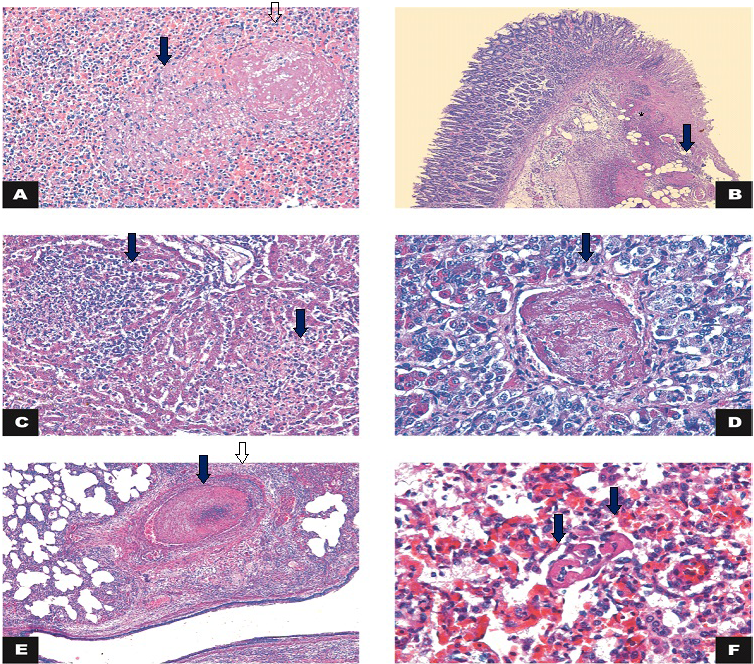
Figure 2: Histology
A) Spleen: necrosis (arrow) associated with thrombosis (open arrow) (calf 3). HE 200x; B) Large intestine: intersection of intact and fibrinonecrotic mucosa associated with multifocal thrombosis (arrow) and inflammatory infiltrate in the lamina propria/submucosa (*) (Calf 1). HE 50x; C) Liver: necrosis and lymphohistiocytic inflammatory infiltrate (arrows) (Calf 2). HE 200x; D) Hypophysis: thrombosis (arrow) (Calf 2). HE 400x; E) Lung: thrombosis (arrow) and vasculitis (arrowhead). HE 50x; F) Lung: capillary thrombosis (arrows) (Calf 4). H&E 400x.
Table 2: Histology findings of the four calves necropsied
|
Histology findings |
Calves |
|
Neutrophilic and lymphohistiocytic broncho-interstitial pneumonia associated with lymphocitic vasculitis and tromboses in the capillaries |
1, 2, 3 and 4 |
|
Vasculitis, necrosis and thrombosis in the liver, gallbladder, spleen and hypophysis |
1, 2, 3 and 4 |
|
Fibrinonecrotic and neutrophilic colitis associated with thrombosis |
1 |
in the body including the liver, spleen, lung and intestine, which results in neutrophilic inflammatory infiltrates.
The mild elevation of AST and GGT enzymes associated with a decrease in albumin and globulins occurred due to hepatic dysfunction and probably reflect parenchymal necrosis. The hypoglycemia seen in calf 3 was due to a gradual reduction of food intake. Furthermore, the liver could not do gluconeogenesis because it was inflamed and necrotic (Santos et al., 2002; Silva et al., 2010). In sum, these observations demonstrate that the clinical follow-up of calves infected with Salmonella is extremely important because the observed clinical signs and the hematological and biochemical alterations progress and change during the course of Salmonella infection.
The pulmonary congestion and the petechiae in various organs are important gross lesions typically observed in Salmonella infections (Silva et al., 2010); this indicates septicemia. Other findings include diffuse catarrhal hemorrhagic fibrinonecrotic ileitis and tifilocolitis, edematous mesenteric lymph nodes, cholecystitis, and erosions on the abomasal mucosa (Gelbert, 2001). These changes were observed in necropsied calves, highlighting the importance of necropsy of animals for determining the diagnosis.
Salmonella species have a lipopolysaccharide (LPS) cell wall that stimulates an inflammatory process. The LPS contains a lipid with endotoxic capacity called “lipid” A, which is released after bacterial death. This cell wall component of the bacterium stimulates the production of cytokines such as interferon, tumor necrosis factor (TNF), interleukin 1 that contribute to thrombosis and vascular changes. This component is also associated with tissue damage, hyperthermia, disseminated intravascular coagulation (DIC) and circulatory collapse ultimately resulting in endotoxic shock (Mohler and House, 2009). These are features seen in the calves reported here.
Microscopic lesions (vasculitis, thrombosis and necrosis) were observed in the liver, kidney, lung and pituitary gland though they varied in severity. Studies with experimental inoculation and natural infection (Marques et al., 2013) demonstrate that S. dublin infection results in these changes.
The resistance to several antimicrobials (Hur et al., 2012), survival for months in the environment in the presence of organic matter and the occurrence of carrier animals that can shed the bacteria for months (Nielsen, 2013) reinforce the importance of the correct diagnosis, treatment and prevention. In cases of suspected infection with Salmonella spp. the identification of the pathogen and antibiotic susceptibility test are essential to implement prophylactic measures.
In disease outbreaks, it is necessary to identify the etiologic cause especially if Salmonella infection is involved because it can have impacts on the entire herd with virulence diversity due to serotypes and varied clinical manifestations. Diagnosis of infection with Salmonella spp. is also fundamental to assess the need for implementation of vaccination against bacterial agent.
Conflict of interest
The authors declare that they have conflict of interest.
AUTHORS CONTRIBUTION
JAZ, FMC, RMCG, MBH, and APL prepared the manuscript; the bacteriological examination was done by FMC, MVPS, and MBH; RMM, PPN, AUC and EJFF examined the calves and collected the tissue and blood samples; LAS and FLMM performed the blood tests; RMCG and MVLM were responsible for microscopic analysis. All authors have read and approved the final manuscript.
References





