Research Journal for Veterinary Practitioners
Case Report
Case Report: Chronic Diarrhea in Bubalus bubalis due to Balantidiasis
Abdullah Iqbal1*, Muhammad Tayyab Farooqi2
1District Diagnostic Laboratory, Livestock and Dairy Development Department, Government of Punjab, Mandi Bahauddin, Pakistan; 2District Diagnostic Laboratory, Livestock and Dairy Development Department, Government of Punjab, Gujrat, Pakistan.
Abstract | A 5-year old buffalo was presented for anorexia and watery mucoid diarrhea from two months with the previous history of treatment with anthelmintics and antidiarrheal by local veterinarians. To find the cause of the disease fecal sample was examined and blood was tested for recording hematological parameters. Balantidium coli trophozoites were observed in the fecal sample. Buffalo was treated with oxytetracycline at 10mg/kg body weight for four days. The efficacy of treatment was estimated 10 days post-treatment by the absence of cyst or trophozoites in feces, improvement in hematological parameters and disappearance of clinical signs.
Keywords | Buffalo, Pakistan, Diarrhea, Balantidium coli, Oxytetracycline
Received | August 07, 2020; Accepted | August 22, 2020; Published | November 01, 2020
*Correspondence | Abdullah Iqbal, District Diagnostic Laboratory, Livestock and Dairy Development Department, Government of Punjab, Mandi Bahauddin, Pakistan; Email: abdullahiqbal09@yahoo.com
Citation | Iqbal A, Farooqi MT (2020). Case report: chronic diarrhea in bubalus bubalis due to balantidiasis. Res J. Vet. Pract. 8(4): 48-50.
DOI | http://dx.doi.org/10.17582/journal.rjvp/2020/8.4.48.50
ISSN | 2308-2798
Copyright © 2020 Iqbal and Farooqi. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Balantidium coli (B.coli) is a zoonotic protozoan parasite that belongs to the family of Balantidiidae and causes Balantidiasis (Nakauchi, 1999; McLeod et al., 2015). Human balantidiasis is more common in areas where contaminated water is used for drinking purposes (Cox, 2005). Although pigs are the main reservoir host of B.coli, it has been reported from sheep, goat, cattle, buffalo, camel, horses and cats (Solaymani-Mohammadi & Petri, 2005; Jian et al., 2018). According to different studies around Lahore in Pakistan prevalence of B.coli in buffalo, cattle sheep and goat was 20%, 25%, 3.99% and 3.46% respectively. B.coli has been reported from donkeys from Pakistan with 18.3% prevalence (Bilal et al., 2009; Khan et al., 2013; Jamil et al., 2015). In humans, a 0.7% prevalence of B.coli has been reported from Panama (Schuster & Ramirez-Avila, 2008). Human Balantidiasis cases are also reported from India in recent years (Parija et al., 2016). The primary aim of this study is to describe the therapeutic management of Balantidiasis in buffaloes.
Case History
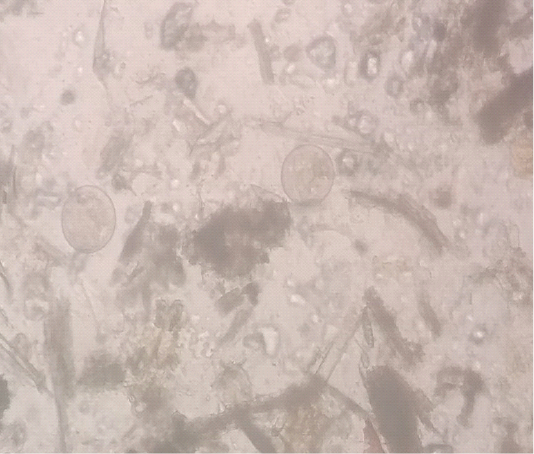
A five-year-old buffalo was presented in District Disease Diagnostic Laboratory, Mandi Bahauddin, Punjab with a history of chronic diarrhea, anorexia, pale mucus membranes and decreased milk yield. The animal was previously treated with anthelmintic and antidiarrheal along with some herbal products. But, the animal showed no positive response. The temperature of the animal was normal. The fecal sample was collected directly from the rectum. Fecal smear was prepared by the sedimentation method. Fecal examination revealed B .coli trophozoites (Figure 1). By using the McMaster technique 700 trophozoites/gram of feces were observed. In an EDTA coated vacutainer 3ml of blood was collected from the jugular vein. Hematology was performed using the Mythic-Vet18 Hematology analyzer. Oxytetracycline was recommended at 10mg/kg bodyweight for four days via the oral route. Buffalo started to pas normal feces from the third day. After 10 days fecal and blood samples were again collected. Feces were free of B .coli trophozoites and hematological parameters were also improving (Table 1).
Table 1: Hematological Parameters before the start of treatment and 10 days post-treatment
|
WBC 103/µl |
LYM 103/µl |
MON 103/µl |
GRA 103/µl |
RBC 106/µl |
HGB g/dl | HCT % |
PLT 103/µl |
|
| Day 0 | 7.4 | 3.7 | 0.3 | 3.4 | 4.28 | 9.2 | 27.6 | 189 |
| 10 days post-treatment | 7.0 | 3.4 | 0.4 | 3.2 | 4.41 | 11.3 | 34 |
223 |
Discussion
B.coli has a wide range of hosts and causes intestinal lesions leading to persistent diarrhea along with weight loss (Bauri et al., 2012). Mostly B.coli infects the caecum and colon and transmitted directly but invasive cases of B.coli are also documented where it perforated the colon mucosa and spread to peritoneal cavity and lungs (Anargyrou et al., 2003). A recent study also revealed the involvement of B.coli in osteomyelitis of the spine (Dhawan et al., 2013).
In this study, buffalo was treated successfully with oral administration of oxytetracycline at 10mg/kg weight dose rate. Tarar et al. (2008) also reported the efficacy of oxytetracycline in B.coli infection in buffalo. The use of oxytetracycline in sheep and goats also decreased B.coli cyst considerably (Jamil et al., 2015). No abnormality in the leukogram was observed in this case. These findings are similar to B.coli infection in camels in Iran where the leukogram of an effected camel was also normal (Tajik et al., 2013). Hemoglobin was below the normal range at day first but 10 days post-treatment hemoglobin was increased. These changes in hemoglobin values were similar to findings of B.coli infection in Sheep, goats and donkeys but dissimilar to B.coli infection in camels (Khan et al., 2013; Tajik et al., 2013; Jamil et al., 2015).
Conclusion
This case report highlights the severity of parasitic diarrhea, the importance of proper diagnosis of the cause of diarrhea and the possibility of intestinal zoonotic infection from domestic animals. In Pakistan, where animals of different species are kept at the same place and peoples don’t follow hygienic practices B.coli infection in children and immunocompromised patients can be life-threatening.
acknowledgements
We are thankful to Dr. Fraz Munir Khan in Livestock and Dairy Development Department, Punjab for his kind guidance at every step that helped a lot in improving this study.
Conflict of interest
There are no conflicts of interest.
authors contribution
AI conceived the idea. MTF participated in write up. Both the authors read and approved the manuscript.
References