Research Journal for Veterinary Practitioners
Case Report
Phenobarbital-Responsive Bilateral Zygomatic Sialadenitis following an Enterotomy in a Cavalier King Charles Spaniel
Paoul S Martinez, Renee Carter, Lorrie Gaschen, Kirk Ryan
Department of Veterinary Clinical Sciences, School of Veterinary Medicine Louisiana State University, Baton Rouge, LA 70803, USA.
Abstract | A 6 year old male castrated Cavalier King Charles Spaniel presented to the Louisiana State University Veterinary Teaching Hospital for a three day history of bilateral acute trismus, exophthalmos, and blindness. These signs developed after an exploratory laparotomy and gastrointestinal foreign body removal was performed. On presentation, the ophthalmic examination revealed bilateral decreased retropulsion, absent bilateral direct and consensual pupillary light responses, bilateral blindness, and a superficial ulcer in right eye. Ocular ultrasound and computed tomography of the skull were performed and identified a bilateral retrobulbar mass effect originating from the zygomatic salivary glands. Ultrasound guided fine needle aspirate cytology revealed neutrophilic inflammation. Fungal testing and bacterial cultures were performed which were negative for identification of an infectious organism. The patient was placed on a 5-day course of antibiotic therapy and the response was poor. Phenobarbital was added to the treatment regimen. Two days following phenobarbital administration, the exophthalmos and trismus began to resolve. Following phenobarbital therapy, ocular position normalized, trismus completely resolved, and patient returned to normal behavior although blindness remained in both eyes. No other neurologic condition occurred. This paper highlights the early use of phenobarbital for the rapid resolution of zygomatic sialadenitis.
Keywords | Ultrasound, Sialadenitis, Bilateral exophthalmos, Biomicroscopy, Neutrophilic inflammation
Editor | Muhammad Abubakar, National Veterinary Laboratories, Islamabad, Pakistan.
Received | November 07, 2017; Accepted | March 25, 2018; Published | August 20, 2018
*Correspondence | Paoul S Martinez, Department of Veterinary Clinical Sciences, School of Veterinary Medicine Louisiana State University, Baton Rouge, LA 70803, USA; Email: martinez.p@ufl.edu
Citation | Martinez PS, Carter R, Gaschen L, Ryan K (2018). Phenobarbital-responsive bilateral zygomatic sialadenitis following an enterotomy in a cavalier king charles spaniel. Res. J. Vet. Pract. 6(2): 14-19.
DOI | http://dx.doi.org/10.17582/journal.rjvp/2018/6.2.14.19
ISSN (Online) | 2308-2798
Copyright © 2018 Martinez et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Exophthalmos, trismus, and decreased retropulsion are common clinical signs exhibited with canines suffering from retrobulbar diseases which can present unilaterally or bilaterally. Differentials for retrobulbar disease includes neoplasia, abscessation, orbital cellulitis, masticatory myositis, and zygomatic salivary disease. Diseases affecting the salivary glands in the canine population are relatively uncommon (Boland et al., 2013) and include neoplasia, sialocoele, sialadenitis, sialolith, and sialadenosis mainly affecting the sublingual salivary glands (Boland et al., 2013; Perez-Ecija et al., 2012; McGill et al., 2009). Sialadenitis is defined as inflammation of the mandibular and/or zygomatic salivary glands and is the principle disorder reported to affect the salivary glands of dogs. Sialadenitis has been reported with trauma, infection, inflammation of surrounding tissues, and immune-mediated disease. Sialadenitis, sialadenosis, and sialocoeles can result from ascending infections, bacteremia, trauma, or ductal stone obstructions (Boland et al., 2013; Wang et al., 2009).
Sialadenitis and sialadenosis have been linked to a peripheral form of epilepsy with observed clinical response to phenobarbital (Schroeder and Berry, 1998). Previous reports demonstrated a complete clinical response to oral phenobarbital with no recurrence of disease once medication was tapered (Boydell et al., 2000; McGill et al., 2009; Schroeder and Berry 1998). One report identified sialadenitis and sialocele involving the zygomatic salivary glands in a dog that developed seizures later in the disease process; these seizures were not controlled with phenobarbital and resulted in euthanasia of the dog (McGill et al., 2009).
Diagnosis of retrobulbar diseases can be challenging due to the anatomy of the orbit being incompletely encased in orbital bones with ligaments and musculature comprising the lateral orbit (Cannon et al., 2011). Diagnostically, the use of ultrasonography, computed tomography with contrast, magnetic resonance imaging, and sialadenography, have been useful imaging modalities for the evaluation of orbital lesions (Boland et al., 2013; Daniel and Mitchell 1999; Dennis, 2000; Penninck et al., 2001; Tremolada et al., 2015). Fine needle aspirates or biopsies of orbital lesions can be obtained for diagnosis. Medical and surgical options are available depending on the underlying cause of the retrobulbar lesion. For abscessation, neoplasia, or sialocele, surgical removal or drainage may be pursued.
This report describes a case of acute onset phenobarbital-responsive zygomatic sialadenitis in a Cavalier King Charles Spaniel diagnosed by computed tomography of the skull.
Case Report
A 6-year-old intact male Cavalier King Charles Spaniel presented to the Louisiana State University School of Veterinary Medicine Teaching Hospital for acute trismus, exophthalmos, and blindness. The patient originally presented to his referring veterinarian for a one day history of vomiting. Two days after initial presentation, abdominal radiographs were performed and a gastrointestinal foreign body was noted. An exploratory laparotomy was performed and tire mulch was removed from the duodenum and the colon. No significant post-operative complications were noted within three days of the surgery. On the fourth day, the patient was noted to have focal swelling ventral to the eyelids beginning with the left side of the face. Bilateral exophthalmos and pain upon opening his mouth were also noted. At this time, he maintained vision in both eyes based on a positive menace response performed by his primary care veterinarian but had no direct pupillary light reflex (PLR) of the left eye. The primary care veterinarian performed fine needle aspirates via an oral approach under sedation. Retrobulbar bulbar aspirates were performed just caudal to the last upper molars using a 22-gauge needle. Cytology interpreted prior to referral revealed toxic neutrophils with no organisms identified. By that evening, the patient became blind and absent direct and consensual PLRs were observed in both eyes. The dog was placed on unknown dosages of intravenous ampicillin twice daily and enrofloxacin once daily. After two days without clinical improvement, the dog was referred for further diagnostics and treatment. Prior to the vomiting episode, the dog was reported to be normal with no neurologic, ophthalmic, or gastrointestinal abnormalities noted.
On presentation to the referral hospital, the dog was lethargic, mentally dull, with bilateral exophthalmos and extreme pain elicited upon opening his mouth. Physical examination revealed a grade III/VI left systolic heart murmur in addition to the trismus. There was mild ventral eyelid swelling and decreased retropulsion noted bilaterally. The facial musculature palpated normally and the submandibular or prescapular lymph nodes palpated symmetrical, soft, and non-painful. Ophthalmic examination included cranial nerve assessment, pupillary light reflexes, menace response, palpebral reflex, dazzle reflex, and Schirmer tear test I (Intervet, Inc. Summit, NJ). Intraocular pressures were measured using applanation tonometry (Medtronic Tono-Pen XL) following instillation of topical anesthetic (Proparacaine, Alcon Laboratories, Inc. Fort Worth, Texas). Fluorescein staining (Contracare Ophthalmics and Diagnostics, Gujarat, India) was performed to evaluate the cornea. Finally, induction of mydriasis (Tropicamide, Akorn, Inc. Lake Forest, IL) was accomplished to facilitate biomicroscopy slit lamp exam for anterior segment evaluation and indirect ophthalmoscopy for fundic evaluation. Ophthalmic examination revealed bilateral exophthalmos, lagophthalmos in both eyes, absent menace in both eyes, absent direct and consensual pupillary light responses (PLRs) in both eyes, and absent dazzle reflex in both eyes. A central horizontal superficial corneal ulcer in the right eye was attributed to the lagophthalmos. Schirmer tear test I results were 14 mm/minute in the left eye and 17 mm/minute in the right eye. The intraocular pressures were 6 mmHg in both eyes and the intraocular examination, including fundic examination was unremarkable. The ventral abdominal skin incision from the laparotomy was healing and appeared unremarkable.
Complete blood count and blood chemistry data demonstrated a stress leukogram, normocytic, normochromic anemia, and limited electrolyte changes. Thoracic radiographs showed mild cardiomegaly and a normal post-operative pneumoperitoneum. Abdominal ultrasound was performed to assess the previous abdominal surgery site and pneumoperitoneum. Mild pneumoperitoneum, suspected regenerative splenic nodules, bilateral renal pylectasia, moderate prostatomegaly, and mild urinary bladder debris were noted.
Ocular ultrasonography with a 12MHz linear array transducer (Toshiba Aplio 300®), under light sedation diagnosed a bilateral retrobulbar mass with normal intraocular structures (Figure 1). Ultrasound guided fine needle aspirates of the retrobulbar masses were performed through the dorsal eyelid under heavy sedation. These aspirates yielded viscous clear fluid and cytology revealed neutrophilic inflammation with no organisms seen. An oral examination was also performed that revealed no abnormalities of either dentition or the soft tissue structures of the mouth. Aerobic and anaerobic cultures of ultrasound-guided retrobulbar aspirates were submitted and yielded no growth. Computed tomography of the head was performed with 1.2mm slices, pre- and post- intravenous contrast administration (Iohexol® , GE Healthcare, Princeton NJ , 300 mg/ml, 2ml/kg body weight) using both a standard soft tissue and a bone algorithm (GE Lightspeed® 16 slice, GE Healthcare, NJ). Bilaterally, the zygomatic salivary glands were confluent with two large hypo-attenuating (16 Hounsfield units pre-contrast), non-contrast enhancing (18 Hounsfield units post-contract), irregularly-marginated, partially septated lobular masses in the retrobulbar space (Figure 2 and 3). The left gland measured 4.9 x 2.4 x 4.1 cm and the right gland measured 3.4 x 2.2 x 4.1 cm. Both glands were laterally and rostrally deviating the globes. There was mild bilateral enlargement and elongation of the retro-pharyngeal lymph nodes. Additional findings included mild to moderate dilatation of the lateral, third, and fourth ventricles with caudal flattening of the cerebellum.

Figure 1: Ocular ultrasound of the left eye demonstrating a large hyperechoic retrobulbar space occupying lesion
The heart murmur and radiographically apparent cardiomegaly were attributed to breed-related mitral valve disease (Swift et al., 2017). Likewise, cerebellar and ventricular CT findings were compatible with occipital bone malformation reported in Cavalier King Charles Spaniels (Couturier et al., 2008).
Differential diagnoses for the zygomatic salivary disease included abscess (fungal and/or bacterial), sialdenitis, hematoma, neoplasia, and siaolocele (Boland et al., 2013; Cannon et al., 2011; Hammer et al., 2001; McGill et al., 2009; Perez-Ecija, Estepa, and Mendoza, 2012; Wang, Ledbetter, and Kern, 2009). Hematoma and neoplasia were determined to be less likely based on results of fine needle aspirate cytology. Urine enzyme immunoassay (EIA) antigen testing for histoplasma and blastomyces fungal organisms (MiraVista Diagnostics, Indianapolis, IN) and cryptococcal serum antigen latex agglutination testing (Louisiana Animal Disease Diagnostic Laboratory, Baton Rouge, LA) were negative. Zygomatic sialadenitis was considered a likely differential based on the clinical presentation, CT findings, and exclusion of other salivary diseases.

Figure 2: Pre-contrast computed tomographic image in a soft tissue window showing bilateral retrobulbar masses (Z). Both masses are hypodense and poorly defined and larger on the right (left side of image).
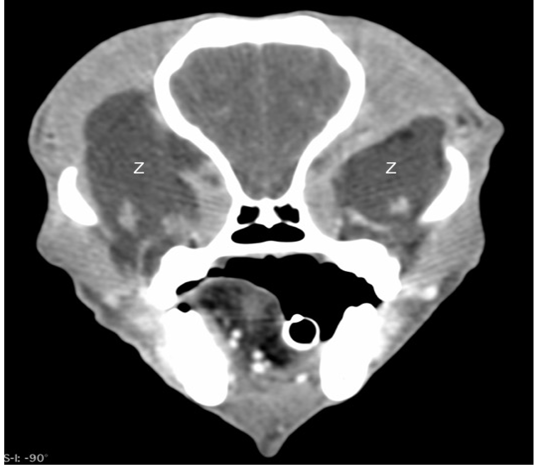
Figure 3: Post-contrast computed tomographic image in a soft tissue window showing the bilateral retrobulbar masses (Z) to have no enhancement.
Pending the results of diagnostic tests, systemic broad-spectrum antibiotic therapy was continued at our hospital. Antibiotic therapy consisted of intravenous Ampicillin and sulbactam (USP, AuroMedics Pharma, LLC, Dayton, NJ) at a dose of 22 mg/kg IV q. 8 hours) and enrofloxacin (Baytril® Bayer HeatlhCare, LLC, Animal Health Division, Shawnee Mission, KS) at a dose of 10 mg/kg IV q. 24 hours. Ongoing antibiotic therapy was continued due to potential contamination of the orbit with oral bacteria following retrobulbar aspirates performed prior to and after referral. Antibiotic therapy was selected for broad-spectrum coverage for aerobic and anaerobic bacteria. Oral itraconzaole therapy at 5 mg/kg/day orally was initiated while fungal tests were pending. Itraconazole therapy was not extended past a two-week course after fungal testing was confirmed to be negative. For pain management, an intravenous constant rate of infusion (CRI) of fentanyl was initiated at a rate of 3 micrograms/kilogram/hour. Due to the dog’s persistently depressed mentation, opioids were discontinued 24 hours after initiation. Topical therapy for the corneal ulcer in the right eye (OD) included neomycin-polymyxin B-bacitracin ophthalmic ointment (Vetropolycin® Dechra Veterinary Products, Overland Park, KS) every 4 hours OD. Topical lubricant (Optixcare® lubricating gel) was also applied every 2 hours to both eyes due to lagophthalmos.
Once infectious diseases were excluded, zygomatic sialadenitis was considered the primary differential. This disease is a diagnosed via exclusion of other possibilities and may be responsive to phenobarbital. Phenobarbital at 2mg/kg was given intravenously every 12 hours as an empirical therapy and to prevent seizure activity which has been reported with sialadenitis.
Over a three-day period, the dog became more energetic and significantly less exophthalmic. Trismus improved readily and on day three of hospitalization, he began consuming small meals. The absent vision and PLRs in both eyes remained static during hospitalization. The dog was discharged with minimal exophthalmos, resolving corneal ulceration and no pain upon opening of the mouth.
At the two-week recheck with the primary care veterinarian, the owner reported that the dog showed significant clinical improvement. In particular, there was no pain, the ocular position had returned to normal and a normal appetite had resumed. The dog’s right eye was fluorescein negative at this exam, but bilateral blindness persisted. Oral antibiotics, antifungal medications and ophthalmic medications were discontinued. The primary care veterinarian instructed the owners to discontinue phenobarbital after six weeks of therapy, with no apparent recurrence of signs noted by the owner. At a six-month follow-up visit with the primary care veterinarian, the dog remained blind in both eyes but had regained direct and consensual PLRs of the left eye. He appeared comfortable, with no additional residual signs noted.
Discussion
This report describes the presence of phenobarbital responsive bilateral zygomatic sialadenitis which resulted in clinical signs consistent with retrobulbar disease (including exophthalmos, decreased ocular retropulsion, and trismus). Zygomatic salivary disease is an uncommon cause of retrobulbar disease (Boland et al., 2013; Perez-Ecija, Estepa, and Mendoza 2012; McGill et al., 2009; Hammer et al., 2001). The differential diagnosis for zygomatic salivary disease, includes neoplasia, sialocele, sialadenitis, and sialoliths (Boland et al., 2013; Perez-Ecija, Estepa, and Mendoza 2012; McGill et al., 2009).
In this case, no evidence of primary or metastatic neoplasia was identified during a thorough clinical investigation which included body cavity imaging and computed tomography of the skull. Fine needle aspirates of bilateral retrobulbar lesions revealed viscous clear fluid compatible with saliva and cytologic evidence of neutrophilic inflammation. No infectious etiology was cytologically evident and a thorough search for local and systemic infectious agents was negative. CT of the skull determined the bilateral retrobulbar masses to be zygomatic in origin with impingement on the caudal aspect of the globe and continuing along the extent of the retrobulbar optic nerve.
The blindness in this case is attributed to progression of the zygomatic salivary gland disease, causing compression of the optic nerve. However, blindness may also have resulted from an extension of inflammation along the optic nerve. Notably, bilateral fine needle aspirates of the retrobulbar spaces were performed prior to referral and it is possible that these procedures precipitated damage to the optic nerves. However, since the blindness subsequently observed was bilateral, an iatrogenic cause is considered less likely.
Most studies describing zygomatic salivary gland diseases report the use of systemic corticosteroids. In our case, corticosteroid treatments were not pursued due to the recent enterotomies performed in the dog (Boydell et al., 2000; Dennis, 2000; McGill et al., 2009; Perez-Ecija et al., 2012; Schroeder and Berry. 1998) and the risk of incisional dehiscence.
In contrast to other reported cases of zygomatic sialadenitis, our patient’s response to antibiotic therapy at the referring clinic and our hospital was disappointing and was marked by no change in clinical signs. In other reports, zygomatic sialadenitis has been treated with longer antibiotic therapy usually consisting of treatment for 2-4 weeks. Another contrasting feature of our case is a lack of systemic anti-inflammatory medications used in our patient. Due to the potential for an infectious etiology and the presence of a recent abdominal surgery, systemic anti-inflammatory medications were not recommended.
In this case, clinical improvement began two days following initiation of phenobarbital therapy. Phenobarbital was used in our case based on previous reports of phenobarbital responsive sialadenitis and sialadenosis in dogs (Boydell et al., 2000; McGill et al., 2009; Schroeder and Berry, 1998). Our case included many features similar to these published cases, including the presence of exophthalmos, pain upon opening of the mouth, blindness, identified retrobulbar mass on ocular ultrasound, identification of zygomatic salivary gland enlargement on CT, and neutrophilic inflammation noted cytologically.
Phenobarbital was continued for two weeks following resolution of clinical signs and ultimately discontinued by the primary care veterinarian. Other than remaining bilaterally blind, the dog was unaffected neurologically.
Although zygomatic salivary gland disease is an uncommon cause of retrobulbar disease in dogs, consideration for the disease is important as a potential differential diagnosis. We believe that the early administration of phenobarbital in our case resulted in rapid resolution of most clinical signs attributed to bilateral zygomatic sialadenitis in this dog. The persistent blindness in our patient may be a result of the amount and duration of the compression of the optic nerve and the extension of chronic inflammation to the optic nerve. Early resolution of exophthalmos, optic nerve compression, and inflammation of the optic nerve is important to optimize the opportunity to regain vision. For cases of zygomatic sialadenitis early initiation of phenobarbital may be beneficial for resolution of clinical signs and may potentially delay or avoid the onset of seizure-like activity.
Authors Contribution
Drs. PSM and KR were responsible for the initial evaluation, work-up and treatment of the case. Dr. RTC provided ophthalmic examination and interpretation. Dr. LG conducted and reviewed the advanced imaging obtained. Dr. PSM drafted the initial manuscript and all authors contributed to the revisions for the final manuscript.
References





