Journal of Animal Health and Production
Research Article
Effect of Incubation Duration on Broiler Breeder Eggs Hatchability and Post-Hatch Performance
Adnan Yousaf1, Adnan Jabbar1, Imdad Hussain Leghari2*, Muhammad Abbas3
1Sadiq Poultry (Pvt) Limited, Chakri Hatchery Rawalpindi, Pakistan; 2Department of Poultry Husbandry, Sindh Agriculture University, 70060 Tandojam, Pakistan; 3Faculty of Animal Husbandry and Veterinary Science, Sindh Agriculture University Tandojam, Pakistan.
Abstract | Incubation duration is most important factor to achieve standard hatchability, water loss and chick yield. The current study was conducted to evaluate the exact duration of egg incubation and its effects on egg hatchability and broiler’s performance at farm. Eggs from Ross-308 breeder flocks having age of 42-46 weeks and standard weight of 55-60g were divided into two experimental groups each consist of (n= 538,560) eggs. Group A was incubated for 506 hours (444h in setter and 62 h in hatcher) and hatch pulling was performed twice 1st after 494 h and remaining un-hatch eggs were again shifted to hatchers for next 12 h for 2nd pulling (conventional method of hatch pulling in Pakistan). For group B, hatch pulling was performed after 506 h (456 h in setter and 50 h in hatcher) and complete hatch pulling was done only once. Eggs weight (54.9 ±0.6, 53.9±0.8)at transfer (from setter to hatcher), water loss at transfer (10.6±0.7, 11.67±0.7) and chick weight at day one (42.7±0.3, 41.6±0.3) were significantly (P<0.05) different between group A and B respectively. Similarly, hatchability percentage (85.16±1.02,85.56±1.02) and dead in shell (DIS) percentage(6.62 ±1.5, 6.61±0.8)were also positively (P<0.05) changed in group A as compared to group B respectively. Mortality (3.47±0.23, 2.28±0.06), weight gain (1955.66±25.02, 2001.33±24.33), feed intake (3260.51±13.47, 3245.02±18.03,) and feed conservation ratio at day 35(1.716±0.03, 1.44±0.02) were also found significantly (P<0.05) different in group A than B respectively. These, results indicated that incubation of eggs for 506 h along with single hatch pull is better in terms of water loss, hatchability, DIS percentage and post-hatch performance of broilers.
Keywords | Broiler, Incubation duration, Hatchability, Post-hatch performance
Editor | Asghar Ali Kamboh, Sindh Agriculture University, Tandojam, Pakistan.
Received | March 19, 2017; Accepted | September 26, 2017; Published | October 30, 2017
*Correspondence | Imdad Hussain Leghari, Department of Poultry Husbandry, Sindh Agriculture University, 70060 Tandojam, Pakistan; Email: imdadleghari@hotmail.com
Citation | Adnan Y, Adnan J, Imdad HL, Abbas M (2017). Effect of incubation duration on broiler breeder eggs hatchability and post-hatch performance. J. Anim. Health Prod. 5(4): 127-131.
DOI | http://dx.doi.org/10.17582/journal.jahp/2017/5.4.127.131
ISSN (Online) | 2308-2801; ISSN (Print) | 2309-3331
Copyright © 2017 Yousaf et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Poultry is the 2nd largest industry of Pakistan, whose play a key role in GDP of country (Hussain, et al. 2015). Poultry farming is widely adopted in Pakistan and almost every farmstead keeps some poultry mainly for consumption and cash sales. The science and technology have contributed widely for the expansion of poultry industry and a number of strategies have been adopted to enhance the poultry production (Al-Nasrawi, 2016; Aguihe et al., 2017). In Pakistan, there are about 25000 poultry farms, providing employment and income for livelihood of fifteen thousand people. In the country, there are about 400 hatcheries, 150 feed mills, 8.5 million broiler breeders, 0.428 million layer breeders and their feed consumption is 5.51 million metric tons per year (Anonyms, 2011; FAO, 2011).
The studies have declared that hatching egg quality and incubation conditions significantly influence the post-hatch performance of broilers (Almeida et al., 2006; Jabbar and Yousaf, 2017; Yousaf et al., 2017). A number of incubation parameters are known to influence hatchability including the length of incubation, and storage temperature (Tona, et al. 2003), incubator temperature (Yildirim et al., 2004), position of eggs placement in tray (Van de Ven et al., 2011a), turning and turning angle (Tona et al., 2005). Moreover, gaseous exchange and CO2 concentration also affect the hatchability parameters (Everaert et al., 2007).The incubation period of chicken (Gallus gallus) embryo is approximately (506 h) 21.08 days including drying down, and the gap among first to last chick hatch time is approximately 12 to 24 h. (Tong et al., 2013; Van de Ven et al., 2011b). This time interval between first and last chick hatch is called “Hatch Window” (Molenaar et al., 2011). Pulling of the chicks from hatchers is started when almost 90-95% chicks are complete dry (Joseph and Moran, 2005). In commercial hatcheries incubation times of chicken is approximately 504 h (Almeida at el., 2006). However, in some large hatcherieschicks pulling are extended up to 510 to 526 h (Laughlin at el., 2007). Whereas, in Pakistan, traditional method of twice hatch pulling (first at 494 h and second at 506 h) is been used in hatcheries. Keeping in view the above scenario, the current experiment was performed to find out the exact hatch time and its effects on post-hatch performance of broilers.
MATERIALS AND METHODS
Experimental Site
The study was carried out at Sadiq Poultry (Pvt) Limited, Chakri Hatchery Rawalpindi which is situated 5 km from Chakri interchange on motorway (M2). The hatchery contains latest heating ventilation and air conditioning (HVAC) automation. This is the largest hatchery of South Asia, which is producing best quality of chicks through single stage incubation system (Avida G4, Chick Master, USA).
Selection and Handling of Eggs
Eggs (weight 55-60 g) from broiler breeders (Ross-308, 45-50 weeks of age) were divided into two groups, such as A for twice pulling (conventional method) and B single pull. Each experimental group was consisting of n=538,560 eggs, which were graded upon their quality, Poor shell, elongated and cracked eggs were removed, and only standardized eggs were set in the incubator machine (Advida G4, Chick Master, USA) having capacity of 134,640 eggs. These eggs were collected from farm and stored at 20°C and 75% relative humidity until used in hatching trial. Before trial, eggs were fumigated with 20 g KMnO4and 40ml formalin (40%) mixed with 40 ml of water for 100ft3 area for 15 minutes through automatic fumigation process provided by Chick Master.
Incubation Program
The incubation experiment was done quadruplicate and includes (n=134,640) eggs in each experiment. Both groups were pre-heated at 82oF for 5 hours inside incubators. After completion of pre-warming the setter started automatically and goes to the incubation stage profile (Ross prime age recommended by Chicks Master USA). Group A was incubated for 444 h in setter and group B was incubated for 456 h in separate setter. The eggs were then shifted to hatchers, the duration of incubation in hatchers was short for A group (62 h) as compared to B group (50 h).
Hatch Pulling
Hatch pulling for both groups was different. For group A hatch pulling was performed through conventional method of hatch pulling in Pakistan. First pull at 494 h (444 in setters and 50 h in hatchers. For second hatch pull the remaining chicks and unhatched eggs were again shifted to hatcher for next 12 h. After 12 h again pulling of unhatched eggs from group A was performed.
Group B was pulled out only one time after 506 h (456 h in setters and 50 h in hatchers). Nothing was left behind inside hatchers. Hatch pull out was performed through shell separator (KUHL, USA).
Hatchery Analyses
Before transfer to hatchers egg’s water loss of both groups was measured. Water loss was measured for group A after 444 h, while for group B after 456 h using following formula.
Water Loss % = Full tray weight at Setting- Full Tray Weight at Transfer x 100
Full tray weight at Setting- Empty Tray Wight
During transfer from setter to hatchers candling was performed for eggs of both groups through candling tables and results were enumerated in percentage.
Body weight of chicks was determined immediately after hatching through electrical weight balance that was used to estimate the chick yield using following formula. Moreover, grading of chicks was performed on conveyor, an automatic grading table. Only standardized chicks (having shining eyes, soft legs and nose, healed naval and looking healthy) were shifted to chick’s box after counting, while under weight, weak, and unhealed naval chicks were removed as per international standard.
Chick Yield % = Weight of chicks x 100
Egg weight
Dead in shell analysis was done to calculate the embryos death during different stages of incubation. All DIS results were calculated in percentages.
Post-hatch Farming Conditions
After hatching n=56,000 (n=28,000 for each group) day old chicks were sent to Sadiq broiler farm Khilari-Chakri, Rawalpindi to evaluate the post-hatch performance. Environmentally controlled vehicles (75 0F temperatures, 65% humidity) were used to deliver the chicks to the broiler farm. The chicks of both groups A and B were reared in the farm under same housing conditions that includes availability of light (12L:12D; light from 0600 to1800h) along with standardized conditions of temperature, humidity and stocking density (Abel et al., 2014). During whole trial period, chicks of both groups were offered water and feed ad libitum. Sadiq feed was offered to both groups i.e., starter diets from 1 to 12 day (3020 Kcal ME/kg, 22% CP), grower diets from 13 to 22 day (3185 Kcal ME/kg, 20% CP) and finisher diets from 23 to 35 day of age (3230 Kcal ME/kg, 18% CP). The diets were formulated according to the recommendations of the NRC (1994) using windows user-Friendly feed formulation (WUFFDA) software program. Intake of feed and water was record daily, while body weight and total feed consumed were recorded on weekly basis. After 35 days of trial period, chicks weight was measured for both group.
Statistical analysis
All data were analyzed by using Statistical Analysis System package software (SAS version 9.2, SAS Institute Inc., Cary, NC, USA). All means were compared using Duncan’s Multiple Range test and results were presented as mean ± SEM (standard error of mean). Results were considered significant if exist (P< 0.05).
RESULTS AND DISCUSSION
In Pakistan, one hatch pulling after 494 h is a common practice that followed by a shifting of unhatched eggs to same hatchers for next 12 h for second hatch pull. In current study, we have used first time in Pakistan single hatch pull as per international standard (hatch pull only once after 506 h) (Joseph and Moran, 2005).
As shown in Table 1, eggs weight of group A at transfer (54.9 ±0.6) was significantly higher (P<0.05) as compare to group B (53.9±0.8) that probably due to less water loss (P<0.05) in group A (10.6±0.7) than group B (11.67±0.7). Chick weight of group A (42.7±0.3) was significantly high (P<0.05) as compared to chicks from group B (41.6±0.3). The water loss from eggs is a major source of variation in chick quality and weight at day one (Mortola and Gaonac’h-Lovejoy, 2016). According to (Tong et al., 2013) water loss for good quality chicks should be 11-12%,water loss less than 11% causes as cites. So, group B which was incubated for 456 h in setter showed better water loss at transfer.
Hatchability percentage was improved (P < 0.05) in group B (85.56±1.02) as compared to group A (85.16±1.02), while DIS percentage was found lower (P<0.05) in group B (6.61±0.8) as compared to group A (6.62 ±1.5; Table 2). The DIS analysis also revealed that, lowest (P < 0.05) percentage of embryo death was recorded in mid incubation period (8-14 d) as compared to early (0-7 d) and late (15-21 d) stages of incubation (Figure 1). Proper water loss helps to avoid dehydration during transport of chicks from hatchery to farm (Joseph and Moran, 2005). It has also been suggested that pulling of chicks twice disturbs the temperature and humidity for chicks which are under process of hatching that results increased DIS (Van de Ven et al., 2011a).
Table 1: Effect of incubation duration on water loss, chicks yield and chicks weight
| Parameters | Group A | Group B |
|
Egg Weight (g) Day 1st |
60.2±0.7 | 60.1±0.8 |
| Egg weight (g) at transfer |
54.9 ±0.6 a |
53.9±0.8 b |
| Chicks Weight (g) |
42.7±0.3 a |
|
| Water Loss (%) |
10.6±0.7 b |
11.67±0.7 a |
| Chick Yield (%) | 71.54±1.54 |
69.28±0.18 |
a-b denote difference between group A and B at (P< 0.05)
Table 2: Effect of incubation duration on hatchability parameters
| Parameters | Group A | Group B |
| Hatchability (%) | ||
| Candling (%) | 8.23±1.33 | 8.23±0.93 |
| DIS (%)* |
6.62 ±1.5 a |
6.61±0.8 b |
a-b denote difference between group A and B at (P< 0.05)
* DIS: dead in shell
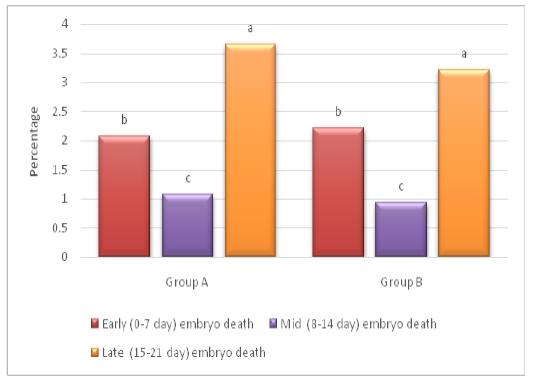
Figure 1: Dead in shell (DIS) analysis on weekly basis a-c denote difference in embryo death between early, mid and late stage of incubation (P< 0.05)
Chicks from both groups were shifted to broiler farm into separate houses and reared for up to 35 days to investigate whether the incubation duration effects on post-hatch performance of broilers. During trial period, mortality, feed intake, weight gain and FCR were recorded and results were presented in Table 3. Interestingly, the effect of 506 h incubation and single hatch pulling on broilers performance was also found better as compared to incubation of 506 hand twice hatch pulling. Feed conversion ratio (FCR) was found significantly better (P<0.05) in B group (1.44±0.02) than A (1.716±0.03). Likewise, Weight gain was improved (P<0.05) in B group (2001.33±24.33) as compare to A (1955.66±25.02). Whereas, feed intake (g/bird) was improved (P<0.05) in group A (3260.51±13.47) than group B (3245.02±18.03) and mortality was reduced significantly (P<0.05) for B (2.28±0.06) as compare to A group (3.47±0.23). The relationship of incubation length and post-hatch performance is poorly understood (Joseph and Moran, 2005). However, these results are in agreement with previous studies that declared that pulling of chicks after whole incubation duration helps chicks grading and improves post-hatch performance (Yousaf, 2016). On the other hand, Joseph and Moran (2005), reported no effect of prolonged holding of eggs in hatchers on post-hatch performance of broilers.
Table 3: Effect of incubation duration on post hatch performance of chicks
| Parameters | Group A | Group B |
| Mortality (%) |
3.47±0.23a |
2.28±0.06b |
| FCR |
1.716±0.03a |
1.44±0.02b |
| Weight gain (g/bird) |
1956.66±25.02b |
2001.33±24.33a |
| Feed in take (g/ bird) |
3260.51±13.47a |
3245.02±18.03b |
a-b denote difference between group A and B at (P< 0.05)
CONCLUSION
In conclusion, the findings of current study tended to show that 506 h incubation along with single hatch pulling to broiler breeder eggs provides better quality chicks and enhances the post-hatch performance. Hence, this method of incubation could be replaced with traditional method of hatch pulling at 494 h that followed by a shifting of unhatched eggs to same hatchers for next 12 h for second hatch pull.
ACKNOWLEDGMENTS
The author’s are thankful to Director of Sadiq Poultry (Pvt) limited Mr. Salman Sadiq for their full support and encouragement during the whole period of research work.
CONFLICT OF INTERESTS
The authors declare that they have no conflict of interest with respect to the research, authorship, and/or publications of this article.
AUTHORS’ CONTRIBUTION
All authors carry equal contribute in this study.
REFERENCES





