Journal of Animal Health and Production
Research Article
In Vitro Combined Effects of Zanthoxylum zanthoxyloides and Newbouldia laevis Methanolic Extracts on Three Life-Cycle Stages of the Parasitic Nematode, Haemonchus contortus
Irvine Yèïnou Minaflinou Sacca Sidi1, Géorcelin Goué Alowanou1, Pascal Abiodoun Olounladé1,2, Vidjinnangni Fifamè Grâce Nadège Dedehou1, Sylvie Mawulé Hounzangbé-Adoté1
1Laboratory of Ethnopharmacology and Animal Health, Faculty of Agronomics Sciences, University of Abomey-Calavi, 01 BP 526 Cotonou, Benin; 2Pluridisciplinary Laboratory, School of Management and Exploitation of livestock Systems, National University of Agriculture, PO Box. 43 Ketou, Benin.
Abstract | Methanolic extracts of Zanthoxylum zanthoxyloides (Fagara), Newbouldia laevis and three of their combinations (50% N. laevis -50% Z. zanthoxyloides, 25% Z. zanthoxyloides -75% N. laevis and 75% Z. zanthoxyloides - 25% N. laevis) were screened in vitro for potential anti-parasitic effects against eggs, infective larvae and adults of Haemonchus contortus. Significant effects were obtained but differences were observed depending on the parasitic stage. The effects of the two plant extracts and their combinations were no dose dependent on egg hatching and infective larvae although more potent effects were usually observed with low concentrations. In contrast, dose–response relationship was found for adult worms. On eggs hatching, the combined extracts 50% N. laevis - 50% Z. zanthoxyloides and 25% Z. zanthoxyloides - 75% N. laevis have showed the same effectiveness like Fagara and N. laevis extracts. Combined plant extracts seemed to be more effective than individual plant extracts on larval migration at less concentrations. Extracts of Fagara and two plant associations (i.e., 50% Z. zanthoxyloides - 50% N. laevis and 75% Z. zanthoxyloides - 25% N. laevis) reduced adult worm motility. Overall, the combination of 50% Z. zanthoxyloides -50% N. laevis was most effective on all three stages of H. contortus. These results showed that Z. zanthoxyloides (Fagara) and N. laevis used in combined form (50%:50%) were more effective on H. contortus thus both plants association could be used as potential alternative of synthetic drugs to control parasitic infections of H. contortus in small ruminants.
Keywords | Zanthoxylum zanthoxyloides, Newbouldia laevis, Haemonchus contortus, Plants combination, Benin
Editor | Asghar Ali Kamboh, Sindh Agriculture University, Tandojam, Pakistan.
Received | September 05, 2016; Accepted | October 20, 2016; Published |October 25, 2016
*Correspondence | Pascal Abiodoun Olounladé, Laboratory of Ethnopharmacology and Animal Health, Faculty of Agronomics Sciences, University of Abomey-Calavi, 01 BP 526 Cotonou, Benin; Email: abiodouno@yahoo.fr
Citation | Sacca-Sidi IYM, Alowanou GG, Olounladé PA, Nadège-Dedehou VFG, Hounzangbé-Adoté SM (2016). In vitro combined effects of Zanthoxylum zanthoxyloides and Newbouldia laevis methanolic extracts on three life-cycle stages of the parasitic nematode, Haemonchus contortus. J. Anim. Health Prod. 4(4): 128-133.
DOI | http://dx.doi.org/10.14737/journal.jahp/2016/4.4.128.133
ISSN | 2308–2801
Copyright © 2016 Sacca-Sidi et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
In tropical developing countries, gastro-intestinal nematode infection remains one of the main causes of impaired production in small ruminants and can lead to fatalities. Haemonchus contortus is one of the most important species because of its high prevalence and pathogenicity (Hounzangbé-Adoté et al., 2005a). This kind of parasite causes adverse effects on the host like haematological and biochemical disturbances (Hoste et al., 2006), loss of body weight (Perry et al., 2002) and huge economic losses (Jabbar et al., 2006). Control of these parasitic infections is generally based on the strategic use of anthelmintic. Unfortunately, these substances are not only less available in the countries of the South but also their cost in local markets is relatively high. In addition, the misuse of these drugs for many years had led to the development of resistant worm strains. Some side effects are noted and the use of disinfectants to control free stage of parasites is harmful to the environment (Wabo-Poné et al., 2011). For these reasons, alternative strategies for nematode control are needed.
In developing nations, traditional methods of controlling nematodes, used by small farmers, remain largely dependent on medicinal plants (Hounzangbé-Adoté et al., 2005a). In this regard, some ethnobotanical investigations have been undertaken everywhere in Africa and particularly in Benin on plants used by breeders to helminthes controlling. Among these plants, Zanthoxylum zanthoxyloides (Fagara), Newbouldia laevis were identified on the basis of a recent questionnaire survey in south of Benin which indicated that they were frequently used by small scale farmers against parasitic infections or to treat associated clinical signs (Hounzangbé-Adoté, 2001). Also sometime, according to Hounzangbé- Adoté (2001) leaves of both plants are used in association to helminthes controlling in this zone of Benin. In earlier studies, Hounzangbé-Adoté et al. (2005a) and (2005b) were assessed the in vitro effects of extracts from these two tropical plants on three life-cycle stages of H. contortus and Trichostrongylus colubriformis. Leaves extract of each plant was significantly reduced from each parasite, eggs hatching, larvae migration and adult worm’s motility. As well, the in vivo effects of fresh and powder leaves of two plants were assessed on sheep and goat infested naturally and artificially with gastrointestinal nematodes parasites (Hounzangbe-Adoté et al., 2005c; Azando et al., 2011a; Minaflinou et al., 2015). Results obtained from these studies showed that these two plants possess anthelmintic properties and confirm their traditional uses by small farmers. However, it is important to verify if both plants used in association will be effective to helminthes controlling according to the investigations from small farmers. The current study was therefore conducted to explore the potential anti-parasitic properties of different combinations of both plants (Z. zanthoxyloides and N. laevis) from Western Africa against H. contortus. In vitro methods were employed targeting three life-cycle stages of H. contortus, i.e. the eggs, the infective larvae and the adult worms.
Materials and Methods
Preparation of Plant Leaves Extracts
Materials screened were leaves of Z. zanthoxyloides (Fag) and leaves of N. laevis (New). Samples of each plant were collected from their natural habitat and dried indoors at room temperature. Z. zanthoxyloides (Lam.) Zepernick and Timler to Rutaceae family and N. laevis (P. Beauv.) Seemann ex Bureau to Bignoniaceae family were identified at national herbarium of Benin respectively under the numbers: AA 6301 /HNB and AA 6302 / HNB. Thereafter, for each plant, 50g of leaves powder were sampled and for both plants combination, respectively 37.5g/12,5g, 25g/25g and 12.5g/37,5g of powdered leaves from each plant was extracted in 500 mL of solvent for two hours at 40°C. A mixture methanol/water was used in proportion to 70:30. Following this, the solution was filtered, and the resulting filtrate was then evaporated in an oven at 47°C. Once the solvent had evaporated, the extract was obtained and dried in a drier for 5 days.
Biological Assays’ Procedures
Extracts effects on the three main stages of the parasite cycle, i.e. the egg, the third-stage larvae (L3s) and the adult worms were measured using different laboratory procedures that is eggs hatching assay, inhibition of larval migration and adult motility inhibition.
Effects on H. contortus Eggs
The method was based on a modification of the egg hatch assay performed to assess anthelmintic resistance by Coles et al. (1992). Briefly, parasite eggs were freshly obtained from donor sheep experimentally infected with H. contortus. The eggs were extracted, washed repeatedly with saline and distributed in 96 multiwell plates at a concentration of 100 eggs /well in 100 µl. Following this, 100 µL of plant association extracts prepared with PBS at different concentrations (75, 150, 300, 600, 1200, and 2400 µg/mL) were added into each well containing eggs solution. A negative control (PBS) and a positive control (Thiabendazole 500, 250 and 125 µg/mL in PBS) were also included. The whole culture plate were then incubated at 27°C for 48 hours. Hatched eggs were counted under a microscope. The test was repeated five times for each plant dose. The percentage inhibition of hatching for each concentration was evaluated microscopically using the modified formula of Coles et al. (1992).
Effects on Infective Larvae
The larval migration inhibition (LMI) bioassay was used as described by Rabel et al. (1994) in order to measure inhibiting activity against infective larvae which were obtained from stool of sheep previously infected artificially with strains of H. contortus. Droppings were collected and left to culture at room temperature for 10 days. The larvae were then extracted from the fecal mass using a Baermann apparatus, which utilizes the water tropism of larvae. After, larvae were incubated for 3 h at 20°C in PBS solutions of plant association extracts, at concentrations of 75, 150, 300 600 or 1200 µg/mL. The larvae were then washed three times in phosphate buffer (PBS) (pH 7.2, 0.15 M) and centrifuged. After the last washing, 800 µL of larvae at a concentration of 1000 L3s/mL was pipetted onto a 20 µm mesh. The sieve was inserted into a conical tube, so that it just touched the surface of the PBS contained therein. Four replicates were run at room temperature for each plant concentration. In addition, negative (larvae incubated in PBS) and positive (larvae incubated in Levamisole at concentrations of 250 µg/mL) controls were run in parallel. After 3 h, the L3s above the sieve were discarded and those which had actively migrated through the mesh into the PBS below, were counted under a microscope at magnification 20X. The percentage of LMI was calculated as:
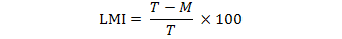
Where;
T is the total number of L3 deposited in the sieve and M the number of L3 having migrated through the mesh into the PBS.
Effects on Adult Worms
The purpose of this assay was to test the anthelmintic effect of the three plant extracts on adult worm motility. This test was performed according to Hounzangbé-Adoté et al. (2005a) and (2005b). Adult worms were obtained from sheep which were experimentally infected with a pure strain of H. contortus. Four weeks after infection, the sheep were euthanized. Immediately after death, the abomasum was collected, opened, briefly washed with saline and placed in a Baermann apparatus with saline at 37°C. After 2 h, the worms that had migrated into the saline were collected and quickly placed in a 48-multiwall plate at a concentration of three worms/ well. The worms were first washed for one hour in PBS with penicillin and streptomycin at a concentration of 4%. Thereafter, 1 mL of the different concentrations of plant association extracts, from 75 to 2400 µg/mL, diluted in PBS were added to the wells. Positive (Levamisole) and negative (PBS plus antibiotic) controls were included on each plate. For each treatment, the measurements were performed on six replicates per dose and per plate. The supernatant was changed every 24 h. The mobility of the adult worms was noted by careful observation under a microscope at magnification 40X after each 6h. At each observation, a motility index was calculated as the ratio between the total numbers of immobile worms/total number of worms, referring in each case to the overall number of worms present in the six replicates.
Statistical Analysis
For assays on egg hatch and larval migration, significant differences in means for the proportion of unhatched eggs and inhibition migration rates between treatments were assessed by the general linear model (GLM) procedures using package MASS (Venables and Ripley, 2002) of R software (R Core Team, 2013). Adult worm assay: for each treatment, the number of immobile worms was recorded with time and survival analyses were assessed using t test of Student. The comparison of the two plants and their combination for each dose was done using Student Newman and Keuls test (SNK) of R software.
Results
Egg Hatch Assay
Z. zanthoxyloides (Fagara), N. laevis extracts and their associations (50%New-50% Z. zanthoxyloides, 25% Z. zanthoxyloides -75% N. laevis and 75% Z. zanthoxyloides -25% N. laevis) have significantly (p < 0.001) reduced in vitro H. contortus eggs hatching. The reduction of eggs hatching rate have varied from 18.84 to 65% following eggs exposure to increasing concentrations (Figure 1). This inhibition was no dose-dependent (p > 0.05) but was function of the plant species tested (p < 0.001). Two plants association extracts 50% N. laevis -50% Z. zanthoxyloides and 25% Z. zanthoxyloides -75% N. laevis have showed the same effectiveness as two plants extracts (Fagara and N. laevis). The association of 75% of Fagara and 25% of N. laevis was the lowest effective (p > 0. 05).
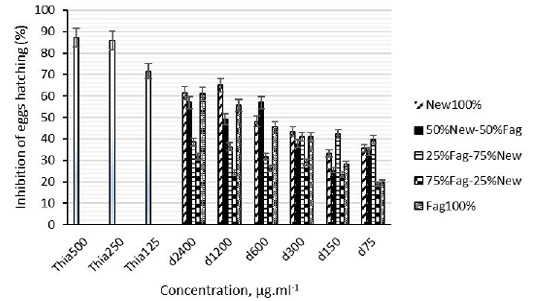
Figure 1: Effects of N. laevis, Z. zanthoxyloides extracts and their different associations extracts (50%New-50%Fag, 25%Fag-75%New and 75%Fag-25%New) on H. contortus eggs hatching. New) Newbouldia laevis; Fag) Zanthoxylum zanthoxyloides; Thia) Thiabendazole
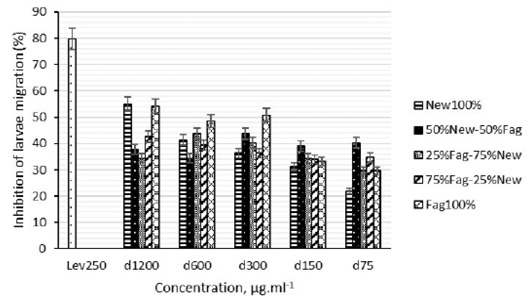
Figure 2: Effects of N. laevis, Z. zanthoxyloides methanolic extracts and their combinations extracts (50%New-50%Fag, 25%Fag-75%New and 75%Fag-25%New ) on H. contortus larvae migration. New) Newbouldia laevis; Fag) Zanthoxylum zanthoxyloides; Lev) Levamisole
Larval Migration Inhibition (LMI)
The inhibition of larval migration induced by Z. zanthoxyloides (Fagara), N. laevis and their associations (50%New-50%Fag, 25%Fag-75%New and 75%Fag-25%New) extracts was neither no dose-depend (p > 0.05) nor the plant species used (p > 0.05) (Figure 2). A significant effect on larval migration was observed for higher (1200 and 600µg/mL) and middle (300µg/mL) concentrations extracts of each plant and each two plant associations. At weak doses, plant association extracts seemed to be more effective than two plants extracts (Figure 2).
Table 1: Effects of various concentrations of methanolic extracts of two plants and their combinations (50%New-50%Fag, 25%Fag-75%New and 75%Fag-25%New) on adult worms’ motility
|
Mobile worms (%) |
|||||
|
Treatment* |
Doses (µg/ml) |
Time (hour) |
|||
|
6h |
24h |
36h |
42h |
||
|
PBS |
00 |
100 |
50 |
25 |
0 |
|
Lev125 |
125 |
100 |
12.5 |
0 |
0 |
|
Lev250 |
250 |
87.5 |
0 |
0 |
0 |
|
Lev500 |
500 |
50 |
0 |
0 |
0 |
|
New 100% |
2400 |
100 |
12.5 |
0 |
0 |
|
1200 |
100 |
25 |
12.5 |
0 |
|
|
600 |
100 |
12.5 |
0 |
0 |
|
|
300 |
100 |
25 |
0 |
0 |
|
|
150 |
100 |
37.5 |
0 |
0 |
|
|
Fagara 100% |
2400 |
87.5 |
0 |
0 |
0 |
|
1200 |
100 |
0 |
0 |
0 |
|
|
600 |
100 |
12.5 |
0 |
0 |
|
|
300 |
100 |
25 |
0 |
0 |
|
|
150 |
100 |
50 |
0 |
0 |
|
|
75%Fag-25%New |
2400 |
100 |
0 |
0 |
0 |
|
1200 |
100 |
0 |
0 |
0 |
|
|
600 |
100 |
0 |
0 |
0 |
|
|
300 |
100 |
25 |
0 |
0 |
|
|
150 |
100 |
37.5 |
0 |
0 |
|
|
50%Fag-50%New |
2400 |
100 |
0 |
0 |
0 |
|
1200 |
100 |
0 |
0 |
0 |
|
|
600 |
100 |
0 |
0 |
0 |
|
|
300 |
100 |
25 |
0 |
0 |
|
|
150 |
100 |
37.5 |
0 |
0 |
|
|
25%Fag-75%New |
2400 |
100 |
0 |
0 |
0 |
|
1200 |
100 |
37.5 |
0 |
0 |
|
|
600 |
100 |
0 |
0 |
0 |
|
|
300 |
100 |
25 |
0 |
0 |
|
|
150 |
100 |
12.5 |
0 |
0 |
|
* PBS: Phosphate Buffered Saline; New: Newbouldia laevis; Fag: Zanthoxylum zanthoxyloides; Lev: Levamisole
Adult Worm Motility
Adult worms’ motility inhibition induced by plant extracts and their combination extracts (50%New-50%Fag, 25%Fag-75%New and 75%Fag-25%New) was dose-dependent (p<0.001) and function of incubation time (p<0.001).
50 percent of worms were immobilized at high concentration of positive control (500 µg/mL) and less of 25 percent at middle dose (250 µg/mL) and high dose (2400 µg/mL) respectively for positive control (250 µg/ml) and Fagara extract, 6 hours after exposure. After 24 hours of incubation, total inhibition was observed in wells of positive control (500 and 250 µg/ml) and those of highest concentrations, 2400 and 1200 µg/mL for Fagara, 2400, 1200 and 600 µg/ml for 75%Fag-25%New and 50%Fag-50%New and 2400 µg/mL for 25%Fag-75%New (Table 1). A strong inhibition of H. contortus adult worms’ motility was observed following exposure to each concentration of plants extracts and their combination extracts after 36 h (Table 1). The motility reduction was total for all extract doses excepted dose 1200 µg/ml of N. laevis extract (Table 1). Nevertheless, after 48 hours of exposure to different concentrations of plant extracts and their association extracts, the reduction of H. contortus motility was total. Extracts of Fagara and two plant associations 50%Fag-50%New and 75%Fag-25%New have in this assay a significant effect on the survival of adult worms of H. contortus (Table 1).
Discussion
Zanthoxylum zanthoxyloides (Fagara) and Newbouldia laevis are present throughout tropical areas of Africa (Bekalo et al., 1996). It has been identified as plants most commonly used by farmers in Benin, as shown by a previous survey (Hounzangbé-Adoté, 2001). According to them the farmers usually used both plants in association for small ruminant gastro-intestinal parasites controlling. Moreover, screening based on in vitro studies using Fagara and N. laevis extracts have provided evidence indicating that different concentrations of extracts modified the viability of both adult worms and third-stage larvae of Haemonchus contortus and Trichostrongylus colubriformis and also affected egg hatching (Hounzangbe-Adote et al., 2005a, b). Recent in vivo studies of Fagara and N. laevis powder leaves realized by Azando et al. (2011a) on same parasites have confirmed both plants anthelmintic properties. However, in this time, no data was not obtained about both plants association effectiveness against nematode infections as indicated by farmers.
The in vitro methods provide a means to screen rapidly for potential anthelmintic activities of the different plant extracts and to analyze the possible mechanisms involved in the interactions between active compounds and parasites. Results obtained from eggs hatching assay indicate that extracts of both plants combination possessed in vitro ovicidal activity. This inhibition was function of the plant species tested and no dose-dependent. Similar observation was done on same plants and parasite in earlier study by Hounzangbé-Adoté et al. (2005a) and recent study by Olounladé et al. (2011). Besides, according to Hounzangbé-Adoté et al.(2005a), the reduction in egg excretion observed in the present study suggest that plants combination extracts could affect the biology of parasitic eggs when sprayed on pastures. By itself, a reduction in egg hatching can also help to modulate the risk of parasitism by limiting the infectivity of pastures grazed by ruminants. The in vitro migration assay is a rapid and effective tool for the determination of drug effects which paralyze nematodes (D’Assonville et al., 1996). The principle of the LMI test depends on an active migration process through the sieve, the reduction in migration rate could be due either to larval mortality or to larval paralysis. These observations were remarked in the present study especially on H. contortus larvae migration. Because a significant reduction in larvae migration rate was observed especially at higher and middle concentration extracts of plant associations. The reduction of adult worms of H. contortus mobility induced by both plants combination extracts was dose dependent and function of time incubation. In this study, a high rates of immobility was recorded after 24 hours of exposure to plant combination extracts, especially plants association 50%Fag-50%New and 75%Fag-25%New. Similar results were reported on adult worms of H. contortus by Hounzangbé-Adoté et al. (2005a) with alcoholic extracts of N. laevis and Z. zanthoxyloïdes. In that study, they recorded high rates of immobility after only six hours of exposure to N. laevis and after 24 hours of exposure to Z. zanthoxyloïdes. As well in others specie, same observations were noticed by Alowanou et al. (submitted) with M. inermis and C. glutinosum extracts on adult worms of same parasite with 100% no mobile worms after 24 hours to highest dose of 2400 µg/mL.
The ovicidal, larvidal and deworming activities of plants combination extracts observed in this study may be attributed to the presence of secondary metabolites such as saponins, alkaloids, tannins and flavonoids (Brunet et al., 2008; Azando et al., 2011b). Bogning et al. (2016) reported that saponins are known to destabilize cell membranes, hence increase cell permeability by combining with membranes associated sterols, which could have significant effect on parasites eggs hatching.
Conclusion
The association extracts 50% N. laevis -50% Z. zanthoxyloides, 25% Z. zanthoxyloides -75% N. laevis and 75% Z. zanthoxyloides -25% N. laevis were effective against eggs, larvae, and adult worms of H. contortus. The combination extract of 50% N. laevis -50% Z. zanthoxyloides was most effective as compared to other combinations and individual plants on at least two stages of the parasite. These results suggested that these plants could be used as alternative to synthetic drugs and accordingly as a great asset for the control of small ruminant’s gastro-intestinal nematodes, particularly H. contortus.
Acknowledgements
Ours acknowledgments was supported by Laboratory of Ethno pharmacology and Animal Health, FSA, UAC for complementary funds through VPMAP-PAES projects. This study would not have succeeded without the role played by Traditional Healers from Benin. We finally thank the anonymous reviewers for their constructive comments on this Manuscript.
Conflict of Interest
There was no conflict of interest from any of Co-authors concerning this work. It was unanimously packaged.
Authors’ Contribution
Irvine Yèïnou Minaflinou Sacca Sidi conducted the Biological assays in accordance with the protocol and wrote the manuscript. Géorcelin Goué Alowanou, monitored the execution of the protocol and participated in the writing of the manuscript. Pascal Abiodoun Olounladé, followed the biochemical part of the protocol, statistical analyses and participated in the correction of the manuscript. Vidjinnangni Fifamè Nadège Dedehou, monitored the execution of the protocol and participated in the writing of the manuscript. Sylvie Mawulé Hounzangbé-Adoté designed the protocol and participated in the correction of the manuscript.
References





