Journal of Animal Health and Production
Research Article
Effect of Pomegranate Peel Extract on some Biochemical and Histopathological Parameters in Experimental Induced Mice with Staphylococcus aureus
Rana Hussein Raheema
Microbiology Department, College of Medicine, Wasit University, Iraq.
Abstract | This study was aimed to evaluate the effect of pomegranate peel extract on serum glucose, creatinine, serum uric acid and serum total protein in normal and Staphylococcus aureus infected mice. Thirty seven milk samples were collected from Al-Kut city from cows suffering acute mastitis. After biochemical tests and API Staph System it was revealed that 19 out of 37 milk samples contained S. aureus. Ethanolic extract of pomegranate peels was prepared and the highest in vitro antibacterial effect was observed by 300mg/mL against S. aureus with 30.00 ± 0.57 mm of the diameter zone. Thirty lactating mice were divided into three groups. Group (1): negative control (Normal). Group (2): positive control, mice were infected by intramammary injection of S. aureus at 7, 15, 21 and 30 days and left untreated. Group (3): mice infected with intramammary injection of S. aureus and treated orally with 300 mg/kg BW of ethanolic extract of pomegranate peel after 48 h of infection for 15, 21 and 30 days. At the end of experiment, blood samples were taken from anaesthetized mice then the mice were killed for histopathological examination and measured the following: serum glucose, creatinine, serum uric acid and serum total proteins. Results showed significant increase (P <0.05) in the levels of serum glucose, creatinine, serum uric acid and serum total proteins in positive control mice as compared with negative control while pomegranate peel treated group showed a significant (P <0.05) decrease in the levels of serum glucose, creatinine, serum uric acid and serum total proteins. Histological section in mammary gland showed clear pathological effects of S. aureus as compared with those treated with pomegranate peel extracts.
Keywords | pomegranate peel extract, Mice, Mastitis, Biochemical parameters
Editor | Asghar Ali Kamboh, Sindh Agriculture University, Tandojam, Pakistan.
Received | December 19, 2015; Revised | January 05, 2016; Accepted | January 07, 2016; Published | February 05, 2016
*Correspondence | Rana Hussein Raheema, College of Medicine, Wasit University, Iraq; Email: firas_rashad@yahoo.com
Citation | Raheema RH (2016). Effect of pomegranate peel extract on some biochemical and histopathological parameters in experimental induced mice with Staphylococcus aureus. J. Anim. Health Prod. 4(2): 42-49.
DOI | http://dx.doi.org/10.14737/journal.jahp/2016/4.2.42.49
ISSN | 2308–2801
Copyright © 2016 Raheema. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Mastitis continues to be the most costly disease of dairy cattle which causes economic losses for both subclinical and clinical stages of the disease (Ruegg, 2003). Staphylococcus aureus is a pus forming, Gram positive bacteria that known as a primary cause of chronic and deep infection of mammary glands which is extremely difficult to be cured. Udder infection results the mastitis (inflammation of udder) that could be in clinical or subclinical stage, however, it leads transitory to permanent blocking of milk ducts (Nazia et al., 2015; Tyagi et al., 2013). Staphylococcal mastitis has been reported as the most costly mammary gland infection of dairy cattle worldwide (Leitner et al., 2011). Staphylococcus aureus considered to be a contagious udder pathogen that spreads within and between cows during milking (Tyagi et al., 2013).
Pomegranate (Punica granatum) peel has been reported as antioxidant and antibacterial properties (Negi and Jayaprakasha, 2003). Punica granatum peels are widely used to treat infections found in human sexual organs, in addition to treat mastitis, acne, folliculitis, pile, allergic dermatitis, tympanitis, scalds, diarrhea, and dysentery (Singh et al., 2002). This study was aimed to evaluate the effect of pomegranate peel extract on some biochemical parameters and histological study in mice experimentally infected with S. aureus isolates recovered from clinical mastitis milk samples.
Materials and Methods
Isolation of Bacteria
A total of 37 milk samples collected from cows suffering from mastitis from different areas of Al-Kut city. All milk samples were centrifuged at 3000 rpm for 15 minute and the precipitate was cultured on blood agar, incubated aerobically at 37°C for 24 h. Diagnosis depend on morphological character and hemolytic pattern by staphylococcus that observed on sheep blood agar and Gram staining. Then cultured on mannitol salt agar, and biochemical tests (catalase, oxidase, urease hydrolysis) were performed. Diagnosis was confirmed by using Api Staph kit. Antibiotic susceptibility test was determined using a disc diffusion method on Mueller Hinton Agar (Baurer et al., 1966). Different antibiotics used were: Gentamicin (GE), ciprofloxacin (CPF), Erythromycin (E), tetracycline (TE), sulfamethoxazole- trimethoprim (SXT), vancomycin (VA), ampicillin (AM), penicillin (PE) and cephalothin (CF).
Extraction of Pomegranate Peels
Pomegranate peels were purchased from local markets in AL-Kut city / Iraq. Pomegranate peels were cleaned, washed and dried in the dark at room temperature and then ground into a powder. A 50g of the powder was dissolved in 500 mL of ethanol and placed on magnetic stirrer for 72 hours. The solution then filtered. The filtrate was put in incubator at temperature of 40ºC until dryness. The extract was kept in dark glass container at 4°C.
In vitro Antibacterial Activity of Ethanolic Extract of Punica granatum Peels
Agar well diffusion method was used to screen the activity of plant extract in vitro and different concentration of pomegranate peel extract (100, 200 and 300 mg/mL) were poured into the wells with DMSO as a control.
Phytochemical Analysis
Ethanolic extracts of pomegranate peel were tested for the presence of several phytochemicals such as alkaloids, tannins, steroids, saponins, flavonoids and carbohydrate
.
Induction of Mastitis
Thirty lactating Balb /c mice were obtained from the national center for drug control and research, Baghdad. Lactating mice challenged with 102 CFU/ gland of S. aureus according to previously described method (Brouillette et al., 2004). Intramammary injection of mice was carried out as described by Chandler, (1970). Group (1): negative control (normal). Group (2): positive control, mice were infected with intramammary injection of S. aureus at 7, 15, 21 and 30 days and left without treatment. Group (3) : mice infected with intramammary injection of S. aureus and treated orally with 300 mg/kg BW of ethanolic extract of pomegranate peel after 48 h of infection for 15 , 21 and 30 days. At the end of experimental period, blood samples were taken from anaesthetized mice then the mice were killed for histopathological examination and measured the following: serum glucose concentration using biosystem chemical kits (Trinder, 1969; Tietz, 1995). Creatinine was determined by using the methods described by Bartels and Bohmer (1971) and serum uric acid was assessed using a commercially available kit (Bio System-SPAIN) (Fossati et al., 1980), while serum total proteins using biosystem chemical kits (Tietz, 1999). All the experimental protocols were approved by the Institutional Animal Care and Use Committee.
Histopathological Study
Mammary gland and other organs were collected at 7, 15 and 30 days fixed in 10 % neutral formalin for histopathological examination.
Statistical Analysis
Statistical analysis was performed using SPSS-21 (Statistical Packages for Social Sciences- version 21). One Way Analysis of Variance (ANOVA) and Least significant differences (LSD) post hoc test was performed (multiple comparisons), to assess significant difference among means. P < 0.05 was considered statistically significant as described by Released (2012).
RESULTS AND DISCUSSION
Results of bacterial isolation showed that milk samples which were collected from cows suffering from mastitis showed positive results for the presence of Staphylococci. The results showed that after 24 hours incubation at 37°C on blood agar, colonies appeared as yellow, round, smooth and glistening with hemolytic properties. Microscopically those were gram positive small spherical cells and grape like. When S. aureus was sub-cultured on mannitol salt agar medium, the colonies produced smooth convex yellowish in color. The results of biochemical tests were: catalase positive, oxidase negative, urease hydrolysis positive. The API Staph System results confirmed that all the 19 isolates were Staphylococcus aureus. Results of antibiotic susceptibility showed sensitive to Gentamicin, ciprofloxacin, sulfamethoxazole-trimethoprim and cephalothin while resistance to erythromycin, tetracycline, ampicillin and penicillin (Table 1). These results are almost in agreement to recent studies (Habib et al., 2015).
Results of phytochemical analysis of ethanolic extract of pomegranate peels give positive result for tannins, flavonoids and carbohydrates as shown in Table 2. Bhandary et al. (2012) showed that the ethanolic extract was more potent than the aqueous extracts probably because the active principles in the plant dissolved more readily in and ethanol was better extracted by a less polar solvent than water.
Table 1: Potential microbial pathogen isolated from dairy cattle and their susceptibility to antibiotics
|
Bacteria |
Antibiotics |
||||||||
|
GE |
CPF |
E |
TE |
SXT |
VA |
AM |
PE |
CF |
|
|
Staph aureus |
S |
S |
R |
R |
S |
I |
R |
R |
S |
R: resistant; S: susceptible; I: intermediate
Gentamicin (GE), ciprofloxacin (CPF), Erythromycin (E), tetracycline (TE), sulfamethoxazole-trimethoprim (SXT), vancomycin (VA), ampicillin (AM), penicillin (PE) and cephalothin (CF).
Table 2: Phytochemical analysis of pomegranate peel extract
|
Chemical compounds |
Ethanolic extract |
|
Alkaloid |
- |
|
Tannins |
+ |
|
Steroid |
- |
|
Saponnins |
- |
|
Flavonoids |
+ |
|
Carbohydrates (Benedict’s test) |
+ |
Table 3: Antibacterial activity of pomegranate peels determined by agar well-diffusion method against S. aureus
|
Concentration |
Inhibition zone Mean±SE |
|
100 |
21.67±0.33c |
|
200 |
25.67±0.33b |
|
300 |
30.00±0.57a |
Means with different letters differ significantly (P< 0.05)
The results of antibacterial activity of pomegranate peels extract showed significant differences between different concentration (P< 0.05) and maximum effect was observed against staphylococcus aureus (30.00 ± 0.57 mm) with concentration of 300 mg/mL as shown in Table 3. The similar finding was also reported by Fawole et al. (2012) who showed that the peel of pomegranate showed strong broad-spectrum activity against Gram-positive and Gram-negative bacteria. The results showed that both Iraqi and Syrian pomegranate juice exhibiting antimicrobial activity against all Gram positive and negative but largest effect was on Staphylococcus aureus bacteria (Abdul Sattar and Hasan, 2012).
The intra-mammary injection of S. aureus effectively induced mastitis as reflected by significance change in biochemical parameters such as significant elevation of blood glucose level in infected group (Table 4) while showed significant (P< 0.05) decreased in pomegranate peel treated group. Prasetyastuti et al. (2014) indicated that pomegranate juice has hypoglycemic and antioxidative effects in STZ induced diabetic rats. The beneficial effects of pomegranate include the cardiovascular protective role, neuroprotective activity, hypoglycemic effect and anticancer properties in particular against prostate, colon and breast cancer (Johanningsmeier and Harris, 2011). Treatment with pomegranate peel extract reduced the elevated levels of serum creatinine (Table 5) and urea (Table 6). Ethanolic pomegranate peel extract restores the renal function by preserving the structural integrity of renal cells against infection with S. aureus, as evidenced by significantly preventing an increase in the levels of serum creatinine and urea. Similar
Table 4: Effect of pomegranate peel treatment on serum glucose levels (mmoL/L) in mouse experimentally infected with S.aureus
|
Period |
Mean±Std. Error |
|
Control |
4.22±0.01d |
|
Infection after 7 days |
3.88±0.01e |
|
Infection after 15 days |
4.76±0.03b |
|
Treated after 15 days |
3.57±0.04f |
|
Infection after 21 days |
4.88±0.02a |
|
Treated after 21 days |
3.33±0.02g |
|
Infection after 30 days |
4.47±0.003c |
|
Treated after 30 days |
3.21±0.01h |
Means with different letters differ significantly (P< 0.05)
Table 5: Effect of pomegranate peel treatment on serum creatinine levels (mg/dL) in mouse experimentally infected with S.aureus
|
Period |
Mean± Std. Error |
|
Control |
.70±0.11f |
|
Infection after 7 days |
2.60±0.07a |
|
Infection after 15 days |
1.82±0.07b |
|
Treated after 15 days |
1.38±0.07d |
|
Infection after 21 days |
1.67±0.07bc |
|
Treated after 21 days |
1.11±0.07e |
|
Infection after 30 days |
1.52±0.07cd |
|
Treated after 30 days |
.82±0.07f |
Means with different letters differ significantly (P< 0.05)
Table 6: Effect of pomegranate peel treatment Serum uric acid level (mg/dL) in mouse experimentally infected with S.aureus
|
Period |
Mean±Std. Error |
|
Control |
4.84±0.02d |
|
Infection after 7 days |
4.69±0.07ef |
|
Infection after 15 days |
5.29±0.03c |
|
Treated after 15 days |
4.73±0.02e |
|
Infection after 21 days |
5.36±0.007b |
|
Treated after 21 days |
4.64±0.02f |
|
Infection after 30 days |
5.43±0.02a |
|
Treated after 30 days |
4.16±0.03g |
Means with different letters differ significantly (P< 0.05)
observation was obtained by Ahmed and Ali (2010) who reported that pomegranate peel ethanol extract is a potent nephropreventive agent.
Results illustrated in Table 7 indicated that the treated group with pomegranate peels was significantly (P< 0.05) decreased in serum total protein (TP) as compared to infected group and control group. The decrease in serum total protein recorded in the current work agrees with the results reported by El Sayed et al. (2014) showed significant decrease in the levels of TP after the ethanolic extracts of olive leaf and pomegranate peel. Osman et al. (2012) showed that total protein was not significantly affected (P >0.05) by any of the treatment of pomegranate peel and juice on rats treated with alloxan-induced diabetes.
Table 7: Effect of pomegranate peel treatment on serum total proteins level (g/L) in mouse experimentally infected with S.aureus
|
Period |
Mean±Std. Error |
|
Control |
64.49±0.04g |
|
Infection after 7 days |
69.50±0.01e |
|
Infection after 15 days |
80.32±0.01a |
|
Treated after 15 days |
75.79±0.007c |
|
Infection after 21 days |
76.57±0.02b |
|
Treated after 21 days |
70.49±0.02d |
|
Infection after 30 days |
66.51±0.003f |
|
Treated after 30 days |
61.70±0.01h |
Means with different letters differ significantly (P< 0.05)
Histopathological Change
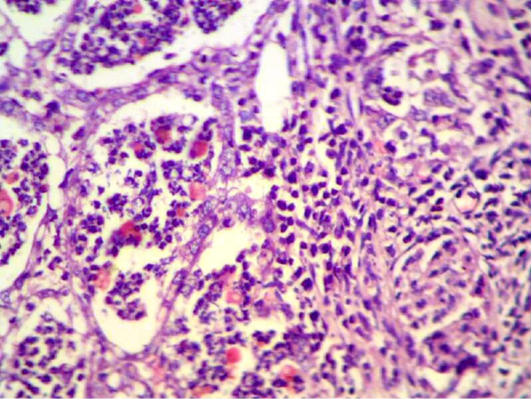
After one week of S. aureus infection intra mammary gland revealed severe destruction of mammary gland with abundant of inflammatory cells infiltration and also showed abscess formation inside the mammary alveoli. In other section, mammary gland shows severe inflammatory cell infiltration, hemorrhagic exudates (oedema), destruction of adipose tissue and mammary alveoli (Figure 1, 2 and 4). AL-Nakeeb (2011) reported that Staphylococcus aureus infection in lung can cause edema, hemorrhage and necrosis with thickening of the wall of alveoli, formation of interstitial exudates, infiltration of inflammatory cells and hemorrhage. Lam et al. (1963) and Cheng et al. (2009) reported that the responsive invasion of immune cells to the site of infection is accompanied by liquefaction necrosis and the release of pus with replicating staphylococci into circulating body fluids. S. aureus is a pyogenic pathogen capable of tissue invasion and evasion of phagocytosis by neutrophils which involved in the inflammatory response that leads to septic shock (Sriskandan and Cohen, 1999).
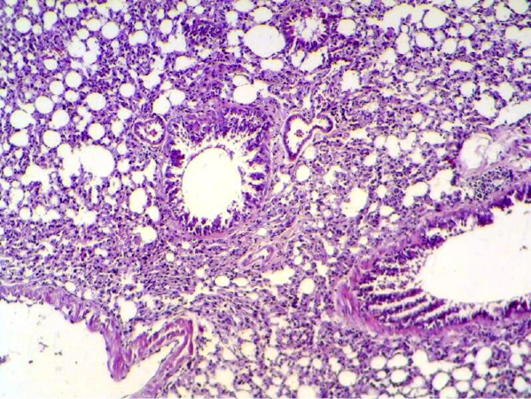
Histopathology of lung tissue of mice infected with
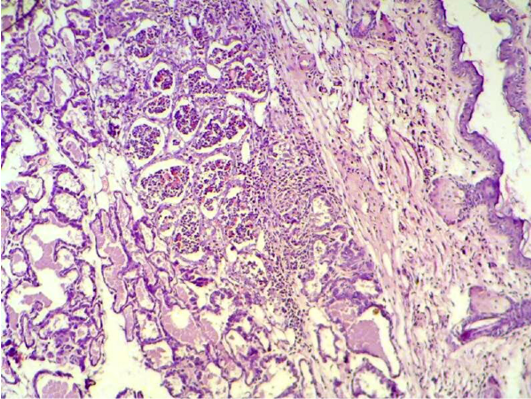
Figure 1: Mammary gland section shows sever destruction of mammary gland with abundant of inflammatory cells infiltration (H&E 200 X)
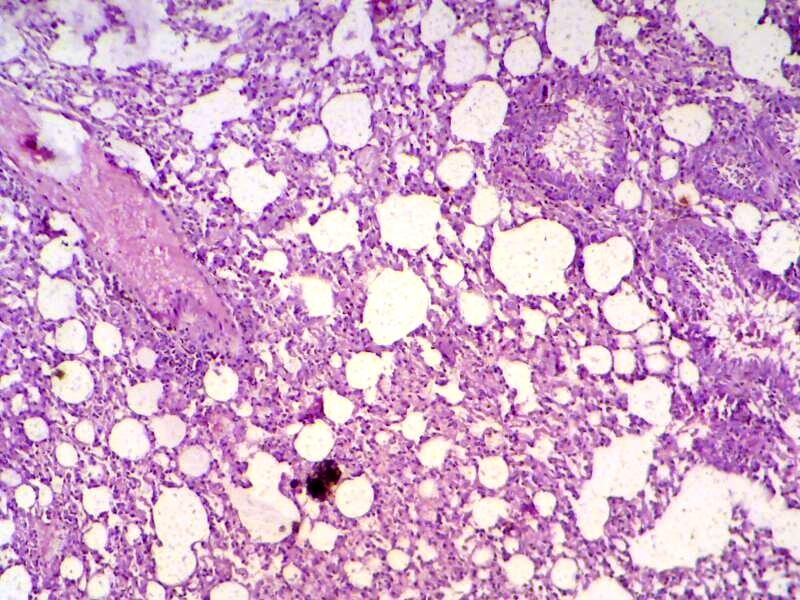
Figure 3: Lung section shows sever inflammatory reaction around the small bronchial (bronchiolitis) congestion and destruction of the alveoli. (H&E 200 X)
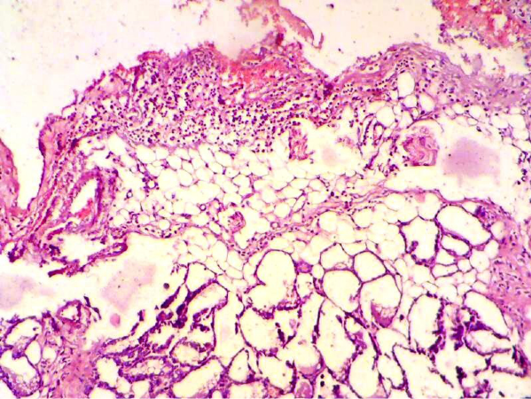
Figure 4: Mammary gland section shows sever inflammatory cell infiltration, haemorrhagic exudate (oedema) and destruction of adipose tissue and mammary alveoli (H&E 200 X)
S. aureus showed severs inflammatory reaction around the small bronchial (bronchiolitis) congestion and destruction of the alveoli (Figure 3). In liver, it showed depletion of glycoprotein granules with degeneration and necrosis of hepatocyte cell (Figure 5 and 6) also in lung shows damage of alveoli septa and small bronchial epithelial (Figure 7). Staphylococcus aureus infection causes tissue damage as characterized by inflammatory polymorphonuclear cell infiltration and necrosis of hepatocytes, granulomatous inflammation in the lungs narrowing of the pulmonary alveolar septa and infiltration of inflammatory cells occurs, abscesses and skin necrosis (Fauzi et al., 2012).
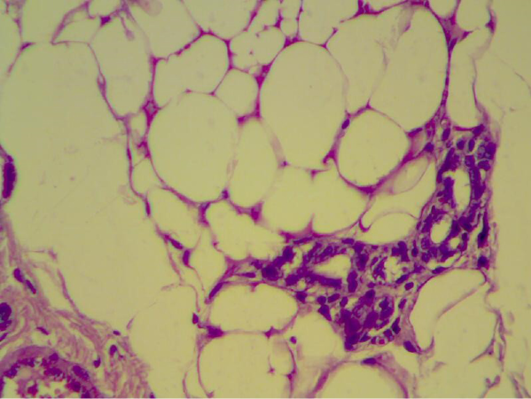
Also liver section obtained from animals after two weeks of infection with S. aureus showed congestion with degeneration and depletion of glycoprotein granules (Figure 8).
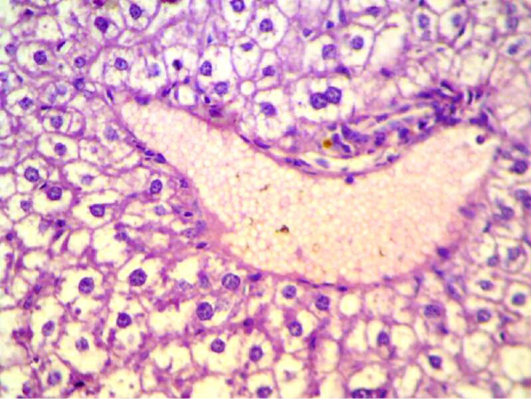
Figure 5: Liver section shows depletion of glycoprotein granules with degeneration and necrosis and inflammatory cell infiltration (H&E 400 X)
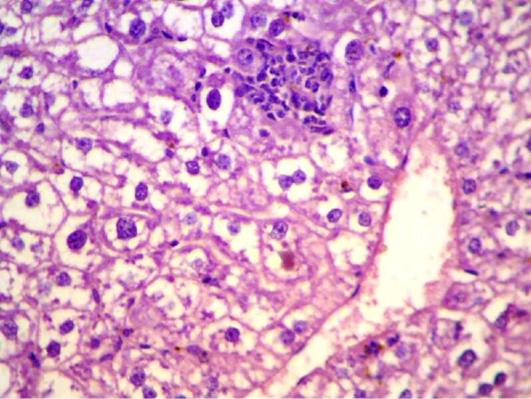
Figure 6: Liver section shows depletion of glycoprotein granules with degeneration and necrosis and inflammatory cell infiltration (H&E 200 X)
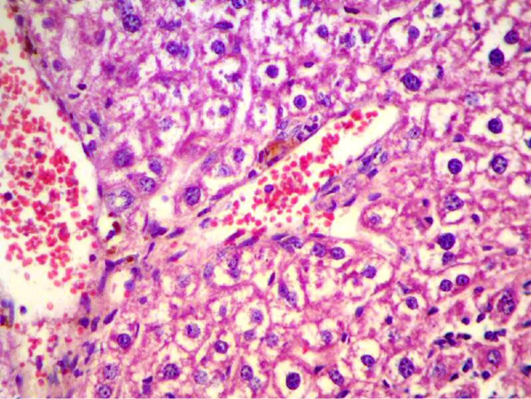
Figure 8: Liver section shows congestion with degeneration and depletion of glycoprotein granules (H&E 400 X)
Manasathien et al. (2012) found that pomegranate peel and seed extracts were rich in phenolic and flavonoid compounds with potentially high antioxidant activities, less cytotoxicity to normal cells, high anti-proliferation against cancerous cells. In kidney section obtained from animals after four weeks of infection with S. aureus intramammary injection showed there was degeneration, necrosis in renal tubules and inflammatory cell infiltration (Figure 9). Staphylococci that disseminate through the bloodstream enter peripheral tissues and seed infectious lesions, initially inducing inflammatory responses that attract neutrophils, macrophages, and other phagocytes (Lowy, 1998).
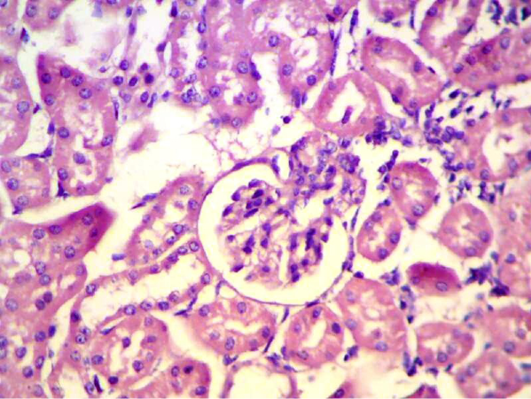
Figure 9: Kidney section shows degeneration, necrosis in renal tubules and inflammatory cell infiltration. (H&E 400 X)
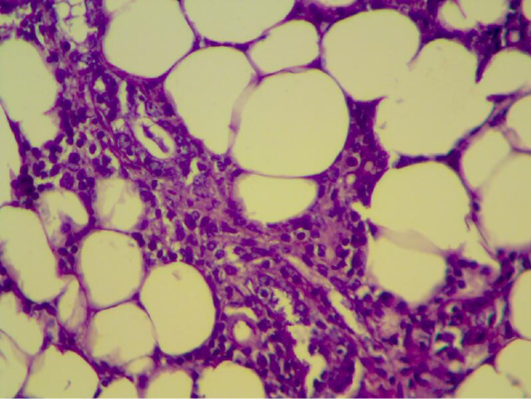
Figure 10: Mammary gland section shows inflammatory reaction around mammary alveoli with destruction of adipose tissue. (H&E 200 X)
Histopathology of mammary gland of mice were infected with S. aureus and treated with pomegranate peels showing inflammatory reaction around mammary alveoli with destruction of adipose tissue (Figure 10). In 2nd week it seems to be look – like normal appearance of mammary tissue (Figure 11 and 12) and also in 4th weeks. Braga et al. (2005) showed that pomegranate peels extracts inhibit or delay Staphylococcus aureus growth and subsequent enterotoxin production at a low extract concentration bacterial growth was delayed, and at a higher concentration growth was eliminated. The ethanolic extract of P. granatum showed antibacterial activity against S. aureus (Machado et al., 2003). The tannin rich ellagitannins and phenolic acids of Punica granatum have antibacterial, antifungal and antiprotozoal activity (Supayang et al., 2005; Vasconcelos et al., 2003).
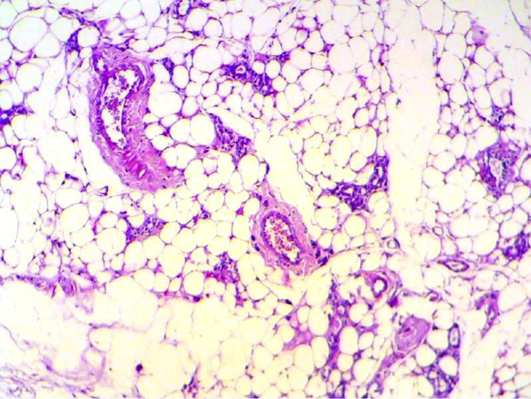
Figure 12: Mammary gland section shows mild inflammatory cell with normal looking adipose tissue. (H&E 200 X) (H&E 400 X)
In conclusion, the extract of Punica granatum peels have antibacterial and protective roles against S. aureus accompanied with significant effect on biochemical parameters and histopathological changes.
aCKNOWLEDGEMENT
The author highly acknowledged Dr. Firas Rashad Al-Samarai for his assistance in data analysis and publishing this paper.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES