Journal of Animal Health and Production
Research Article
Seroprevalence of Brucellosis in Holstein-Friesian and Indigenous Cattle Breeds of Sindh Province, Pakistan
Mazhar Hussain Mangi1, Asghar Ali Kamboh1*, Rahmatullah Rind1, Parkash Dewani2, Zaheer Ahmed Nizamani4, Ali Raza Mangi3, Ali Raza Nizamani4, Waseem Ali Vistro3
1Department of Veterinary Microbiology, Faculty of Animal Husbandry and Veterinary Sciences, Sindh Agriculture University, Tandojam; 2Directorate of Veterinary Research & Diagnosis, Central Veterinary Diagnosis Laboratory (CVDL) Tandojam, Sindh; 3Department of Veterinary Anatomy and histology, Faculty of Animal Husbandry and Veterinary Sciences, Sindh Agriculture University, Tandojam; 4Department of Veterinary Pathology, Faculty of Animal Husbandry and Veterinary Sciences, Sindh Agriculture University, Tandojam, Pakistan.
Abstract | Brucellosis is considered to be one of the most widespread zoonosis in the world. A comparative study on the seroprevalence of brucellosis in indigenous cattle breeds and exotic cattle (Holstein-Friesian) was carried out in district Hyderabad, Sindh, Pakistan. A total of 500 serum samples, 100 each from Red Sindhi, Thari, Kankrej, Bhagnari and Holstein-Friesian breeds were collected from both male and female animals and examined by Rose Bengal plate test (RBPT), serum agglutination test (SAT) and Competitive Enzyme Linked Immunosorbent Assay (c-ELISA). The overall prevalence of brucellosis in cattle was determined as 25, 23.2 and 11.8% by RBPT, SAT and c-ELISA respectively. However, highest prevalence of brucellosis was recorded in Holstein-Friesian (RBPT: 35%; SAT: 33%; c-ELISA: 17%), followed by Red Sindhi (RBPT: 28%; SAT: 26%; c-ELISA: 15%), Thari (RBPT: 24%; SAT: 23%; c-ELISA: 9%), Kankrej (RBPT: 20%; SAT: 18%; c-ELISA: 10%) and Bhagnari (RBPT: 18%; SAT: 16%; c-ELISA: 8%). The sex-wise comparison of seropositive animals indicated that the prevalence of brucellosis was 26.88% in females and 8% in males by both, RBPT and SAT. Furthermore somewhat lower prevalence (12.22%) of brucellosis was demonstrated in female cattle by c-ELISA. The effect of age on the seroprevalence of brucellosis in cattle of Sindh province was also studied. Of the 250 sera examined from cattle ≤ 4 years of age, 51(20.4%), 49(19.6%) and 26 (10.4%) were found positive by RBPT, SAT and c-ELISA respectively. While, of the 250 sera of ˃ 4 years of age 74 (29.6%), 66 (26.4%) and 33 (13.2%) were found positive by RBPT, SAT and c-ELISA, respectively. It was concluded from the present study that exotic cattle (Holstein-Friesian) have higher seroprevalence of brucellosis compared to local cattle breeds amongst which highest prevalence was recorded in Red Sindhi and lowest in Bhagnari breed. Further, among the sero-diagnostic techniques applied during present investigation, the c-ELISA was found as superior technique to determine brucellosis in cattle.
Keywords | Seroprevalence, Brucellosis, Holstein-Friesian, Cattle breeds, Sindh
Editor | Sanjay Kumar Singh, In-Charge Animal Gynecology Lab, Animal Reproduction Division, Indian Veterinary Research Institute, Izatnagar 243122, Bareilly (UP), India.
Received | July 13, 2015; Revised | August 30, 2015; Accepted | September 02, 2015; Published | September 29, 2015
*Correspondence | Asghar Ali Kamboh, Sindh Agriculture University, Tandojam, Pakistan; Email: drasgharkamboh@yahoo.com
Citation | Mangi MH, Kamboh AA, Rind R, Dewani P, Nizamani ZA, Mangi AR, Nizamani AR, Vistro WA (2015). Seroprevalence of brucellosis in Holstein-Friesian and indigenous cattle breeds of Sindh Province, Pakistan. J. Anim. Health Prod. 3(4): 82-87.
DOI | http://dx.doi.org/10.14737/journal.jahp/2015/3.4.82.87
ISSN | 2308–2801
Copyright © 2015 Mangi et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Brucellosis is considered to be one of the most widespread zoonosis in the world (Schelling et al., 2003). It is an important reproductive disease that causes significant reproductive losses in sexually mature animals (Forbes and Tessaro, 1996; Wadood et al., 2009). According to OIE, it is the second most important zoonotic disease in the world that affects cattle, swine, sheep, goats, cattle and dogs (OIE, 2008). The disease is manifested by late term abortions, weak calves, still births, infertility and characterized mainly by retained placenta, epididymitis and orchitis with excretion of the organisms in uterine discharges and milk (Buhari et al., 2015; England et al., 2004). The disease is known to cause morbidity and considerable losses of productivity (Pappas, 2006). The disease is important from economic point of view, because it is one of the most devastating trans-boundary animal disease and also a major barrier for trade (Gul and Khan, 2007).
Brucellosis is a bacterial disease that caused by the organisms of genus Brucella. These are non-motile, anaerobic, intracellular pathogens (Jarvis et al., 2002) that are usually found in the reticulo-endothelial and reproductive systems. Brucella replicate and persist in cells often resulting in infection or carrier state and also impair innate and adaptive immunity (Fichi, 2003). At least six species of the genus Brucella are recognized to be pathogenic for livestock worldwide. Among these, Brucella abortus is known as causative agent of bovine brucellosis (Corbel, 1998).
For the diagnosis of brucellosis in farm animals, bacteriological, serological and molecular methods are used. However, among these bacteriological and molecular tools like polymerase chain reaction are not widely used due to time consuming and economic issues, respectively. However, serological tests like milk ring test, Rose Bengal plate test etc., are most economical approaches, hence used widely for screening and monitoring of brucellosis in dairy cattle (Ali et al., 2013).
Cattle have been found more resistant to bovine brucellosis than buffaloes (Kamboh et al., 2007). Moreover, inter-breed differences in susceptibility to brucellosis are also reported for both cattle and buffaloes (Ali et al., 2013). However, no information is available about the susceptibility level of indigenous cattle breeds of Sindh province of Pakistan for brucellosis. Therefore, the present study was designed to investigate the seroprevalence of brucellosis in four indigenous and one exotic cattle breeds of Sindh province using three serological techniques.
MATERIALS AND METHODS
Collection of Blood Samples
A total of 500 blood samples were collected from different cattle breeds, i.e., 100 each from Red Sindhi, Thari, Kankrej, Bhagnari and Holstein-Friesian. These were collected from peri-urban private farms and taken from both males (n=10) and females (n=90) from district Hyderabad, Sindh, Pakistan. The blood samples from the animals were obtained through jugular vein by using disposable sterilized plastic syringes. The blood samples were transported in cool chain to the laboratory where the sera were separated by centrifugation at 200 g for 15 minutes. The sera were stored at –20˚C until analysed for Brucella antibodies.
Rose Bengal Plate Test (RBPT)
For Rose Bengal plate test (RBPT), stained Brucella antigens (strain-99) were purchased from Veterinary Research Institute (VRI), Lahore. The antigens were used according to manufacturer’s instructions and test procedure was adopted as described by Morgan et al. (1978). In brief, a drop of serum sample and a drop of Rose Bengal antigen were added in a well of the porcelain plate. The contents of both drops were mixed thoroughly and the reaction was observed after four minutes. Complete agglutination was recorded as positive and matched with positive and negative controls for confirmation.
Serum Agglutination Test (SAT)
Serum agglutination test (SAT) was performed using the procedures described by Stemshorm et al. (1985). In brief, 0.8 ml of phosphate buffer saline (PBS) containing 0.5% phenol was added in clear glass tubes of approximately 2 ml volume. A 0.2 ml of test serum was added to first tube, mixed and then 0.5 ml was transferred to the next tube. After mixing well, 0.5ml was transferred to the third and so on up to the last fifth tube. An equal volume (0.5 ml) of standardized B. abortus antigen with phenol saline dilution (1:20) was added and the tubes were incubated overnight at 37˚C. The results of agglutination in SAT test tubes were determined by reading the degree of sedimentation in the tubes. A titre of 1:40 or more was considered as positive, titre of 1:20 was considered a doubtful and the titre of 1:10 was treated as negative.
Brucella c-ELISA Antibody Test
In this assay, an ELISA kit (SVANOVIR Brucella-Ab I-ELISA, Sweden) was used according to manufacturer instructions. Briefly, a 4 µl of positive and negative control serum were added to selected wells coated with Brucella antigen. An equal volume (4 µl) of serum sample was added to the selected well coated with Brucella antigen. The plate was thoroughly shacked, sealed and incubated at 37˚C for one hour. Then rinsed 3 times with PBS-Tween buffer. A 100 µl of Horse reddish peroxidase (HRP) conjugate was added to each well and incubated at 37˚C for 1 hour. The plate was again rinsed thrice, and 100 µl substrate solution (Tetra methyl benzidine) was added to all wells. The plate was incubated for 10 minutes, at 25˚C. Finally, reaction was stopped by adding 50 µl of stop solution to each well and mixing thoroughly. The optical density (OD) of the control and test sample wells was measured at 450 nm using a micro plate photometer (Thermo Electron, Finland). The OD was measured within 15 minutes after adding the stop solution to prevent the fluctuation in the OD values. All results were calculated in terms of PP (Percentage Positivity) value and interpreted accordingly. The sera sample showing PP value lesser than 15, was considered negative and those showing PP value equal or greater than 15, was known as positive. All the samples were run in duplicates.
Table 1: The seroprevalence of brucellosis within gender of various cattle breeds determined by various techniques
|
Sex |
Test |
Samples |
Breeds |
|||||
|
Holstein-Friesian |
Red Sindhi |
Thari |
Kankrej |
Bhagnari |
Overall |
|||
|
Female* |
RBPT |
Positive No. (%) |
33(36.66) |
27(30.00) |
24(26.66) |
19(21.11) |
18(20.00) |
121(26.88) |
|
SAT |
Positive No. (%) |
32(35.55) |
25(27.77) |
23(25.55) |
17(18.88) |
15(16.66) |
112(24.88) |
|
|
c-ELISA |
Positive No. (%) |
16(17.77) |
13(14.44) |
8(8.88) |
10(11.11) |
8(8.88) |
55(12.22) |
|
|
Male** |
RBPT |
Positive No. (%) |
2(20.0) |
1(10.0) |
0(00.0) |
1(10.0) |
0(00.0) |
4(08.0) |
|
SAT |
Positive No. (%) |
2(20.0) |
1(10.0) |
0(00.0) |
1(10.0) |
0(00.0) |
4(08.0) |
|
|
c-ELISA |
Positive No. (%) |
2(20.0) |
1(10.0) |
0(00.0) |
1(10.0) |
0(00.0) |
4(08.0) |
|
RBPT: Rose Bengal plate test; SAT: serum agglutination test; c-ELISA: Competitive Enzyme Linked Immunosorbent Assay; * Number of samples analyzed in each test = 90; ** Number of samples analysed in each test = 10
Table 2: The seroprevalence of brucellosis within different age groups of cattle breeds determined by various techniques
|
Age* |
Test |
Samples |
Breeds |
|||||
|
Holstein-Friesian |
Red Sindhi |
Thari |
Kankrej |
Bhagnari |
Total |
|||
|
≤4 years |
RBPT |
Positive No. (%) |
12(24.00) |
10(20.00) |
14(28.00) |
08(16.00) |
07(14.00) |
51(20.40) |
|
SAT |
Positive No. (%) |
11(22.00) |
10(20.00) |
13(26.00) |
08(16.00) |
07(14.00) |
49(19.00) |
|
|
c-ELISA |
Positive No. (%) |
07(14.00) |
06(12.00) |
04(08.00) |
06(12.00) |
03(06.00) |
26(10.40) |
|
|
˃4 years |
RBPT |
Positive No. (%) |
23(46.00) |
18(36.00) |
10(20.00) |
12(24.00) |
11(22.00) |
74(26.60) |
|
SAT |
Positive No. (%) |
22(44.00) |
16(32.00) |
09(18.00) |
10(20.00) |
09(18.00) |
66(26.40) |
|
|
c-ELISA |
Positive No. (%) |
10(20.00) |
09(18.00) |
05(10.00) |
04(08.00) |
05(10.00) |
33(13.20) |
|
RBPT: Rose Bengal plate test; SAT: serum agglutination test; c-ELISA: Competitive Enzyme Linked Immunosorbent Assay; * Number of samples analysed in each test = 50
n= 100 for RBT, SAT and c-ELISA; n=500 for total; RBPT= Rose Bengal plate test; SAT= Serum agglutination test; c-ELISA= Competitive Enzyme Linked Immunosorbent Assay
Data analysis
All results are expressed in percentages that were calculated by dividing the number of positive samples with total number of samples x100, using Microsoft Office Excel 2010.
RESULTS
Overall Seroprevalence of Brucellosis in Various Cattle Breeds
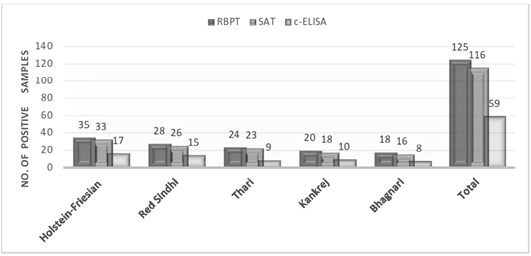
Among 500 tested samples 125, 116 and 59 sera were found positive for brucellosis through RBPT, SAT and c-ELISA respectively (Figure 1). The percentage prevalence of brucellosis was found as 25, 23.2 and 11.8% by RBPT, SAT and c-ELISA respectively. When breeds were compared with each other, highest prevalence of brucellosis was recorded in Holstein-Friesian (RBPT: 35%; SAT: 33%; c-ELISA: 17%), followed by Red Sindhi (RBPT: 28%; SAT: 26%; c-ELISA: 15%), Thari (RBPT: 24%; SAT: 23%; c-ELISA: 9%), Kankrej (RBPT: 20%; SAT: 18%; c-ELISA: 10%) and Bhagnari (RBPT: 18%; SAT: 16%; c-ELISA: 8%).
Seroprevalence of Brucellosis within Gender of Various Cattle Breeds
A total of 50 serum samples from male and 450 from female animals were analysed by RBPT, SAT and c-ELISA to record the gender-wise seroprevalence of brucellosis in cattle and data were presented in Table 1. The prevalence of brucellosis was detected 26.88% by RBPT, 24.88% by SAT and 12.22% by c-ELISA in female animals. However, similar level of prevalence was observed in males i.e., 8.00% by all three techniques.
Seroprevalence of Brucellosis within Different Age Groups of Cattle Breeds
Generally all techniques detected higher prevalence of brucellosis in animals with approximate age of >4 years whereas lower prevalence of brucellosis was observed in cattle ≤4 years of age (Table 2). Among 250 examined sera samples from cattle ≤4 years of age, 51 (20.4%), 49 (19.6%) and 26 (10.4%) were found positive by RBPT, SAT and c-ELISA respectively. On the other hand, when 250 sera were collected and tested from cattle >4 years of age, 74 (29.6%), 66 (26.4%) and 33 (13.2%) were found positive by RBPT, SAT and c-ELISA respectively. Interestingly, among young animals (≤4 years) highest seroprevalence of brucellosis was recorded in Thari cattle by RBPT (28%) and SAT (26%). While, among older animals (>4 years) highest seroprevalence of brucellosis was recorded in Holstein-Friesian cattle by all techniques, i.e., RBPT: 46%; SAT: 44% and c-ELISA: 20%.
DISCUSSION
During present study 500 serum samples were collected from five different local and exotic cattle breeds of Pakistan and were examined by RBPT, SAT and c-ELISA for prevalence of brucellosis. The overall seroprevalence of brucellosis in cattle was determined as 25, 23.2 and 11.8% by RBPT, SAT and c-ELISA, respectively. Anka et al. (2013) recorded the bovine brucellosis in Peninsular, Malaysia, between 2000 and 2008 based on serological data and were detected as 21.8% seropositive cases. While, Cadmus et al. (2013) determined the prevalence of bovine brucellosis based on RBT and c-ELISA as 31.6% and 15.8%, respectively. Whereas Bayemi et al. (2009) conducted a study on Holstein cattle of a small scale dairy production system for Brucella abortus antibodies in Cameroon by ELISA and found a general seroprevalence of 8.4% in Holstein cattle. These differences in seroprevalence of bovine brucellosis might be due to geographical variations, or difference in the animal species (cattle or buffalo) used in study (Ansari et al., 2014; Sachan et al., 2013). Likewise, in a recent study by our laboratory, regarding prevalence of bovine brucellosis, it was found that Brucella abortus specific antibodies have higher ratio in buffalo (47/100) than cattle (31/100) (Soomro et al., 2014). We have also documented the geographical variations in our previous studies (Kamboh et al., 2007; Durrani et al., 2015) for prevalence of bovine brucellosis that might be of nutritional or managemental origin.
Our study have investigated first time, the inter-breed differences for seroprevalence of brucellosis in different local and exotic breeds of cattle. We found highest prevalence of B. abortus antibodies in exotic breed i.e., Holstein-Friesian cattle followed by Red Sindhi, Thari, Kankrej and Bhagnari. These results indicated that our local cattle breeds have resistance for Brucella infection compared to exotic breed. However, this finding needs further investigation especially using the advanced molecular approaches. In similar study in Bangladesh, seroprevalence of brucellosis was reported to be 6.28% in cross breed cattle comparing with 0.85% in local breeds (Sikder et al., 2012). On the contrary, Omer et al. (2000) reported higher prevalence of brucellosis among mixed breeds of cattle compared to indigenous breeds in Asmara region of Eritrea.
During present investigation, we have also compared the diagnostic techniques i.e., RBPT, SAT and c-ELISA for their sensitivity. The results indicated that the highest prevalence of brucellosis was detected by RBPT and SAT in all five breeds of cattle. However, RBPT and SAT have been reported to produce false positive results due to cross reaction with antibodies of other bacterial species whereas in c-ELISA there is much lesser probability of both false positive or negative results (OIE, 2008). While we recorded prevalence rate of 26.88% by RBPT which is similar to those of Anka et al. (2013), Cadmus et al. (2013) and Kamboh et al. (2007); who reported 21.8%, 31.6% and 31% seroprevalence of brucellosis in cattle by RBPT, respectively. Bayemi et al. (2009) using c-ELISA detected 8.4% seroprevalence of brucellosis that is nearly same as we recorded in our present study by the same technique.
An investigation on the seroprevalence of brucellosis in different sexes of all cattle breeds was carried out. The prevalence of brucellosis was detected 26.88% in females by RBPT and 24.88% by SAT. Furthermore, somewhat lower prevalence of brucellosis was demonstrated in female (12.22%) cattle by c-ELISA. On the other hand, interestingly, in males all three techniques indicated the similar level of disease prevalence, this might be due to lesser number of samples used in this study as compared to females. All techniques applied during investigation detected 2-3 times higher prevalence of brucellosis in females as compared to males. It is evident from the study that females are at higher risk of brucellosis than the males. This might be due to males getting infected from Brucella species that could infect a large number of females. Furthermore, this might be due to opening of cervix during estrus for more than a week gets infected from Brucella bacterial species. A higher seroprevalence of bovine brucellosis in females has also been reported by other studies including Malik et al. (2013) who reported an overall seroprevalence of brucellosis in bovine population as 21.36%, with significant (p<0.01) differences in respect to sex (male, 1.81% and female 28.69%). Similarly, Adamu et al. (2014) also found a higher seroprevalence of bovine brucellosis in females (18.0%) than males (5.0%).
We have also evaluated the effect of age on the seroprevalence of brucellosis in cattle of Sindh province, and found high prevalence in older (≥4 year) cattle regardless of technique. The reason of higher prevalence of brucellosis in older animals might be due to lesser immunity because the cattle are in lactating stage. Higher seroprevalence of brucellosis in older age groups of cattle has been reported by several studies. Junaidu et al. (2006) reported a higher (12.4%) sero-prevalence of brucellosis in cattle with the age ranging from 5.5 -10 years. Abou-Eisha, (2000) also recorded a higher prevalence (3.98%) of brucellosis in animals over 5 years of age. Similarly, Adamu et al. (2014) determined a higher prevalence of 9.5% in animals of 5–6½ years of age while, 1.5% in age band of 1–2½ years. All these reports clearly indicates that brucellosis is more prevalent in older animals as compared to young animals.
CONCLUSIONS
From the present study, it could be concluded that brucellosis is prevalent in all cattle breeds of Sindh province. Prevalence of brucellosis is higher in exotic breed (Holstein-Friesian) as compared to indigenous cattle breeds of Sindh province. Among local breeds, prevalence is highest in Red Sindhi and lowest in Bhagnari breed. Older cattle and females are at higher risk of brucellosis. Among the serological techniques c-ELISA has greater sensitivity and specificity in detection of Brucella antibodies in the sera of cattle than RBPT and SAT.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHORS CONTRIBUTION
Mazhar Hussain Mangi conducted the research work, whereas, Asghar Ali Kamboh, Rahmatullah Rind and Parkash Dewani were the mentor of this project. However, Ali Raza Mangi, Ali Raza Nizamani and Waseem Ali Vistro assisted in data analysis and preparation of this manuscript; and Zaheer Ahmed Nizamani done the proof reading of the article.
REFERENCES