Journal of Animal Health and Production
Research Article
Bluetongue Virus in Morocco from 2004-2012
Drif Kamar1*, Elharrak Mehdi2, Loutfi Chafiqa2, Fassi Fihri Ouafaa1
1Microbiology Immunology and Contagious Disease, Agronomy and Veterinary Institute Hassan II; 2Department of Virology, BIOPHARMA, Rabat, Morocco.
Abstract | Bluetongue (BT) is an infectious, non-contagious disease of domestic and wild ruminants caused by Bluetongue Virus (BTV). The disease is quite prevalent in Morocco where serotypes BTV-1 and BTV-4 were found circulating since 2006. Despite use of attenuated bivalent vaccine, outbreaks are not uncommon each year along with lack of knowledge in geographical evolution of each serotype. Here, in this retrospective study from 2004-2012, we have determined current status of BTV prevalence and its serotypes together with techniques used for virus isolation, its antigen and antibody detection. Overall the prevalence of BTV was found to be 77.61% (728/938) and 76.94% (307/339), respectively. Although, we noticed some variation in year-wise distribution of each serotype particularly BTV- 1 where BTV-4 was more prevalent than BTV-1 (45.77% vs. 40.59%). The study provides a baseline data upon circulating serotypes of BTV in Morocco over the years that will certainly help devising its control strategies in future.
Keywords | Bluetongue, Ruminants, Morocco, Vaccination, Prevalence
Editor | Asghar Ali Kamboh, Sindh Agriculture University, Tandojam, Pakistan.
Received | February 14, 2015; Revised | April 20, 2015; Accepted | April 24, 2015; Published | June 02, 2015
*Correspondence | Drif Kamar, Agronomic and Veterinarian Institute Hassan II, MIMC Department, IAV, Rabat, Morocco; Email: drif.kamar@gmail.com
Citation | Kamar D, Mehdi E, Chafiqa L, Ouafaa FF (2015). Bluetongue virus in Morocco from 2004-2012. J. Anim. Health Prod. 3(3): 48-53.
DOI | http://dx.doi.org/10.14737/journal.jahp/2015/3.3.48.53
ISSN | 2308–2801
Copyright © 2015 Kamar et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Bluetongue virus (BTV) is an arthropod-borne emerging pathogen of wild and domestic ruminants that is closely related to Epizootic hemorrhagic disease virus (EHDV). The virus is transmitted by Culicoides with potentiel to spread rapidly to far off countries (Gollapalli et al., 2012; Maan et al., 2012a; Maan et al., 2012b; Maan et al., 2012c; Rao et al., 2012). The causative agent, Bluetongue virus, is the prototype of genus Orbivirus within the family Reoviridae (Mertens et al., 2005). With segmented genome (Seg-1 to Seg-10) of linear double-stranded RNA (dsRNA) (Mertens et al., 2009), BTV includes 26 distinct serotypes (Mertens et al., 2005; Chaignat et al., 2009; Maan et al., 2011). The disease may occur as acute or sub-clinical form characterized by a febrile response, inflammation and congestion leading to facial oedema and hemorrhages and ulceration of the mucous membranes.
Since 2004, the disease is endemic in Morocco with two different serotypes; BTV-4 in 2004 and BTV-1 in 2006. Though from 2005, necessary prophylactic campaigns using bivalent attenuated vaccine were started, outbreaks even in the vaccinated farms were not uncommon. This vaccine failure may be attributed to the nature of farming systems adapted in Morocco that varies between intensive and extensive farming and allows disease spread through vector, the midges. This retrospective study provides an insight into the geographic spread and evolution of BTV serotypes in Morocco since its first epidemic in 2004.
Materials and Methods
Sample Collection
A total of 1337 BTV suspected samples consisting of sera (339) and blood (938) were collected from different outbreaks since 2004. During the first BT epidemics, sera were received from outbreaks and subsequently processed ELISA, however since 2007, both sera and blood added with anti-coagulant (EDTA) were received from outbreaks. All of samples were collected from sick sheep showing clinical signs suspected of BT (Table 1).
Table 1: Geographic provenance and number of samples collected from 2004 to 2012
|
Origin |
2004 |
2006 |
2007 |
2008 |
2009 |
2010 |
2011 |
2012 |
|
Souss massa draa |
28 |
21 |
49 |
30 |
6 |
|||
|
Gharb-Chrarda-Beni hssen |
9 |
19 |
32 |
20 |
12 |
|||
|
Chaouia-ouardigha |
1 |
7 |
22 |
10 |
||||
|
Marrakech-Tensift-Al haouz |
1 |
6 |
7 |
66 |
46 |
4 |
10 |
|
|
Region de l’oriental |
102 |
1 |
23 |
1 |
6 |
2 |
||
|
Grand Casablanca |
2 |
1 |
1 |
11 |
||||
|
Rabat-Sale-Zemmour-Zaer |
24 |
25 |
9 |
3 |
11 |
6 |
2 |
|
|
Doukkala-Abda |
2 |
|||||||
|
Tadla-Azilal |
1 |
10 |
9 |
7 |
5 |
|||
|
Meknes-Tafilalet |
9 |
1 |
53 |
25 |
66 |
133 |
4 |
6 |
|
Fes-Boulmane |
3 |
6 |
15 |
29 |
69 |
46 |
5 |
|
|
Taza-Al hoceima-Taounate |
4 |
1 |
6 |
5 |
||||
|
Tanger-Tetouan |
9 |
25 |
1 |
10 |
||||
|
Unknown area |
8 |
8 |
30 |
19 |
34 |
25 |
16 |
5 |
|
Totaux |
67 |
119 |
222 |
120 |
297 |
350 |
92 |
70 |
Virus Isolation
Blood samples collected from 2004 to 2009 were inoculated into embryonated chicken eggs (ECE) via yolk sac route. The eggs were incubated for 5 days at 35oC and examined daily. Embryos that died between 36 hrs. to 5 days post infection (PI), showing hemorrhagic lesions, were tested by RT-PCR. From 2009 – 2012, virus isolation was performed on cell culture, VERO and BHK cell lines.
Competitive Enzyme Linked Immunosorbent Assay (cELISA)
Serum samples were tested for antibodies against BTV using a commercial competitive enzyme linked immunosorbent assay (cELISA) kit (IDVET Innovative Diagnostics ID Screen Bluetongue Competition ID-Vet, Montpellier, France) as per manufacturer’s instructions.
Virus Neutralization Test (VNT)
To classify the BTV positive sera according to their serotype, a virus neutralization test (VNT) was carried out (OIE terrestrial manual., 2014). Samples were tested for the presence of neutralizing antibodies. Serial dilutions (1:10) of heat-inactivated sera were incubated for 1 h at 37oC with an equal volume (50 ml) of BTV (100 TCID50 with potential to produce pathological change in 50% of cell cultures inoculated). Approximately 104 BHK-21 cells in 100 mL of medium were added per well and, after incubation for 5 days at 37oC with 5% CO2, the microplate was read using an inverted microscope. Two replicates of each serum were tested with BTV-1 and BTV-4 available in Biopharma Lab, Rabat, Morocco. Neutralizing antibody titers were calculated according to Spearman– Karber’s formula and expressed as the last dilution of serum that neutralizes 100% of cytopathic effect (CPE) Serum samples with a virus neutralization titer inferior to 1.3 log10 were considered positive to that particular serotype, BTV-1 or BTV-4.
Real-time Quantitative Reverse Transcription PCR RT-PCR
Blood samples were collected from 938 local and/or crossbred animals. Samples were centrifuged and blood was kept at −20°C until assayed for the presence of BTV.
Viral RNA was extracted from blood using commercially available High Pure RNA Isolation Kit (Roche, Germany) following the instructions provided with kit. Superscript III Platinum One-Step RT-PCR system (Invitrogen, USA) was used to detect antigen using group specific primers BTV-S5-F1 5’-GGCAACYACCAAACATGGA-3’ and BTV-S5-R1 5’- AAAGTYCTCGTGGCATTWGC-3’.
Reverse transcription and amplification were performed by using a 7300 Real Time PCR System (Applied Biosystems). To provide specific information regarding isolate serotype, the primers for specific serotypes were used to amplify the segment 2, allowed to identify the serotype of each tested sample.
Table 2: Different type of samples used in this study
|
Year |
Numbre of samples |
Sera samples |
Blood samples |
Samples isolated in egg and cell culture |
|
2004 |
67 |
67 |
36 |
|
|
2006 |
119 |
119 |
13 |
|
|
2007 |
222 |
97 |
125 |
13 |
|
2008 |
120 |
12 |
108 |
8 |
|
2009 |
297 |
24 |
273 |
11 |
|
2010 |
350 |
40 |
310 |
9 |
|
2011 |
92 |
7 |
85 |
5 |
|
2012 |
70 |
33 |
37 |
15 |
Results
Based upon RT-PCR, overall prevalence of BTV was found to be 77.61% (728/938) where BTV-4 was more prevalent than for RT PCR we use only blood BTV-1 (45.77% vs. 40.59%). Year wise, interestingly, BTV-1 was detected in all samples (100%) collected during the year 2007 with further decrease in 2011 (46.15%) to complete absence in 2012. Contrary to BTV-1, BTV-4 remains relatively constant throughout except 2007 (Table 3).
Table 3: Prevalence of BTV-1 and BTV-4 using a RT-PCR
|
Année |
BT Positif |
BTV1 |
BTV2 |
|
2007 |
67,2 |
100 |
|
|
2008 |
14,81 |
43,75 |
56,25 |
|
2009 |
53,84 |
52,38 |
47,61 |
|
2010 |
78,06 |
31,40 |
68,59 |
|
2011 |
76,47 |
46,15 |
53,84 |
|
2012 |
78,37 |
100 |
Using ELISA, anti-BTV antibodies were detected in 307 sera with an overall seroprevalence of 76.94% (307/339) in different area of Morocco. VNT showed higher prevalence of antibodies against BTV-1 than BTV-4 (75.89% vs 24.10%). The prevalence was significantly higher during 2004-2007 (72.31%) than during 2008-2012 (27.68%) (Table 4).
Table 4: Prevalence of BTV1 and BTV4
|
Year |
Sera |
BT1 |
BT4 |
|
2004 |
67 |
|
44,77 |
|
2006 |
119 |
96,63 |
|
|
2007 |
97 |
79,38 |
|
|
2008 |
12 |
16,66 |
|
|
2009 |
24 |
68,42 |
31,57 |
|
2010 |
40 |
62,06 |
37,93 |
|
2011 |
7 |
60 |
40 |
|
2012 |
33 |
16,66 |
83,33 |
BTVs from 2004 to 2009 were isolated in eggs while, from 2009 to 2012, they were isolated in cell culture. The isolated strains were tested through RT-PCR to constitute a strains collection for Biopharma lab (Table 6).
Discussion
The Moroccan livestock includes approximately 2.8 million cattle, 17.5 million sheep, 5 million goats and 190.0 camels. Both weather conditions (www.agriculture.gov.ma) and the geographic location of Morocco are the most important factors involved in the emergence of the BT in The Mediterranean Basin; a potential source of BTV for Europe and North Africa (Mellor and Wittmann, 2002). Additionally, the international trade of animals has also a role toward introduction of new viruses across different geographies.
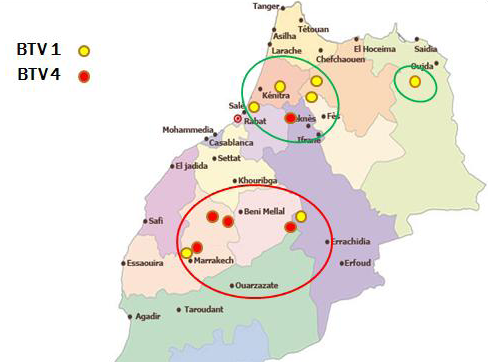
In Morocco, Bluetongue was reported in 50s where serological investigation showed presence of BTV-10. Since then, this area was free until 2004 when BTV-4 appeared in North West of Atlantic coast, and spread to other regions within a few months. In 2006 and 2007, BT re-appeared with serotype 1 that caused severe epidemics in North Africa region. In 2008, numbers of outbreaks were reduced with disease only in cattle sentinels. BT is currently endemic in Morocco and both serotype 4 and 1 have been reported after 2009. This study allowed us to map the distribution of BTV-1 and 4 in order to understand the geographic distribution of each serotype. The outcomes of this study revealed that both serotypes 1 and 4 were geographically present in a separate area but each year the distance between them become shorter. Furthermore, both serotypes (1 and 4) were reported in the same provinces last years. The resurgence of serotype 4 in 2009 has caused increased number of outbreaks with more pronounced virulence signs than those in 2004. Full genome sequencing has revealed that this BTV-4 was a reassortant virus containing fragments of BTV-1 genome (unpublished communication). Although first epidemics of BTV-1 have affected many regions in Morocco, serological and molecular results of this study confirm dominance of BTV-4 mainly after 2009. BTV-1 seems limited in a few areas while BTV-4 was found spreading largely in Morocco (Figure 1). This new epidemiological situation can be attributed to antigenic diversity within BTV serotypes. A co-circulation of both serotypes (BTV1, BTV4) has been confirmed in many areas in Morocco where BTV-4 assigned outbreaks could probably be another re-assortment between BTV-4 and BTV-1, explaining why there is a reduction of BTV-1 assigned outbreaks during the last years.
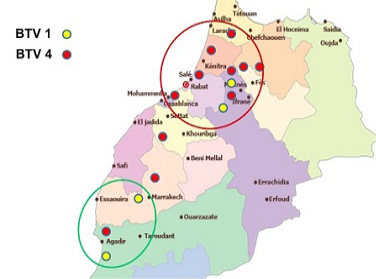
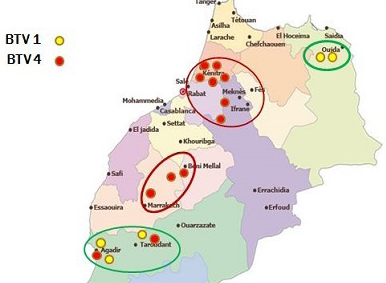
Though many vaccination campaigns were carried out since 2005, both BTV-1 and BTV-4 spread significantly in 2010-2011 (Figure 2 and Figure 3).
Table 5: The provenance of viruses isolated in Morocco (2004-2012)
|
BTV outbreaks |
2004 |
2006 |
2007 |
2008 |
2009 |
2010 |
2011 |
2012 |
|
Souss Massa |
2 |
2 |
2 |
1 |
2 |
|||
|
Marrakech |
4 |
2 |
2 |
2 |
||||
|
Chaouia ouardigha |
3 |
|||||||
|
casa |
2 |
|||||||
|
Rabat-Salé |
6 |
3 |
||||||
|
Gharb |
9 |
3 |
2 |
2 |
2 |
|||
|
Tanger-Tetouan |
9 |
3 |
||||||
|
Oriental |
5 |
3 |
2 |
3 |
||||
|
Meknes |
6 |
2 |
3 |
2 |
2 |
2 |
||
|
Fes |
4 |
2 |
2 |
1 |
2 |
|||
|
Taza-Alhocima |
6 |
|||||||
|
Total |
36 |
13 |
13 |
8 |
11 |
9 |
5 |
15 |
Table 6: PCR serotyping of BTV isolated in cell culture and eggs
|
Year |
virus isolation in cell culture |
virus isolation in eggs |
BTV-1 |
BTV-4 |
|
2004 |
- |
36 |
36 |
|
|
2006 |
13 |
13 |
||
|
2007 |
13 |
13 |
||
|
2008 |
8 |
6 |
2 |
|
|
2009 |
11 |
1 |
4 |
|
|
2010 |
9 |
4 |
5 |
|
|
2011 |
5 |
5 |
||
|
2012 |
15 |
15 |
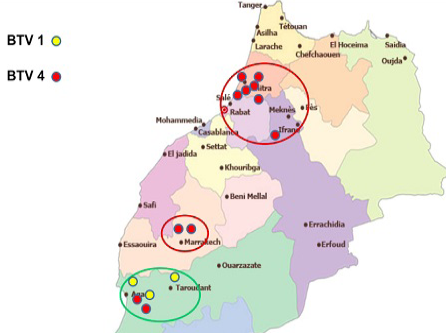
In 2012, except one outbreak of serotype 1 reported in July in the eastern region of the country, virus detected from all reported cases was typed as BTV-4 (Figure 4).
The persistence of BTV in Moroccan livestock remains unclear and the presence of reservoirs were involved several times. Thus, a Moroccan study confirm the role of cattle in maintaining both serotypes 1 and 4, using VN assay (Kamar et al., 2014). In addition to this, circulation of BTV-8 in cattle was also proved by the same study which indicated a silent circulation of this serotype without showing any clinical signs or subsequent economic impact. Moreover in 2010, another full genome sequencing of samples from sheep showed another BTV-4 reassortant that contains segments from serotypes 1, 4 and 8 (unpublished communication). This further explains an increase in prevalence of BTV-4 compared to other serotypes.
Another study showed that domestic dogs seroconvert when infected with BTV. During a BT outbreak in Morocco caused by the same strain of BTV-1 that was used to infect the dogs in an experimental study, a high percentage of the domestic dogs sampled were found to be seropositive for BTV-1 that spread through the ruminant population in the region (Oura et al., 2013). This showed that dogs are capable of being infected with BTV-1, however there lacks data to determine whether dogs mounted a viraemia in response to BTV-1 infection. Another study showed persistence of viral RNA in blood of dogs, which may be an evidence for a low level replication or could be indicative of persistence of viral inoculum (Oura et al., 2011). Likewise, Batten et al. (2011) revealed isolation of BTV from blood of infected camels indicating that they may be capable of transmitting virus to local Culicoides midge population. This suggests that BTV is not depending only to sheep and cattle but canine and camels are also involved in outbreaks at Moroccan region.
The BT outbreaks spread north-west to western Morocco and the evolution of BTV depend upon many factors especially BTV antigenicity, reservoir of viruses and probably silent circulation of other serotypes. Addition to that, vector C. imicola continues to be most prevalent vector mainly with global warming and wind systems reported last years in the Mediterranean region.
Conclusion
In our study, the dominance of BTV-4 was confirmed mainly after 2009 as compared to BTV-1. Distribution of serotypes circulating in Morocco discloses presence of a new BTV-4 which resists all conditions and adapt easily to changes experienced in Morocco. To that end, investigations based upon full genome sequencing should be carried out to clarify the genetic transformation of BTV serotypes isolated in Morocco.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
KD carried out the molecular characterization studies, the serological studies, virus isolation and drafted the manuscript. CL carried out the virus isolation, participated in the serological and molecular studies and helped to draft the manuscript. OFF participated in the design of the study and performed the results analysis. ME participated in the design of the study and coordination. All authors read and approved the final manuscript.
Acknowledgement
We gratefully acknowledge the support of BIOPHARMA Lab, Dr Jamal Malik and Ghizlane Sebbar.
References