Journal of Animal Health and Production
Research Article
Journal of Animal Health and Production. 1 (2): 15–19Incidence and Drug Resistance Pattern of Collibacillosis in Cattle and Buffalo Calves in Western Utter Pradesh in India
Subhash Mailk, Subhash Mailk, Amit Kumar*, Amit Kumar Verma, Manoj Kumar Gupta, Som Dutt Sharma, Arvind Kumar Sharma, Anu Rahal
*Corresponding author: smalikduvasu2008@gmail.com
ARTICLE CITATION:
Mailk S, Kumar A, Verma AK, Gupta MK, Sharma SD, Sharma AK, Rahal A (2013). Incidence and Drug Resistance Pattern of Collibacillosis in Cattle and Buffalo Calves in Western Utter Pradesh in India. J Anim. Health Prod. 1(2): 15–19.
Received: 2013–30–03, Revised: 2013–05–24, Accepted: 2013–05–29
The electronic version of this article is the complete one and can be found online at
(http://nexusacademicpublishers.com/table_contents_detail/11/42/html)
which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Abstract
Diarrhea in farm animals, especially in neonatal calves is one of the most challenging clinical syndromes encountered by practicing large animal’s veterinary practitioners throughout the world. Therefore looking into the intricacy of early age calf diarrhea primarily due to E. coli, the present study was designed to perform the isolation and identification of involved E. coli strains in calf diarrhea and also to establish their drug sensitivity pattern. During the study period total 109 faecal samples were collected from diarrheic cattle and buffalo calves less than three months of age from the university teaching veterinary clinics complex, DUVASU, Mathura, University dairy farm, nearby gaushalas located at Vrindavan and Mathura along with the Veterinary Hospitals of western Utter Pradesh located in district Muzaffarnagar, Baghpat, Meerut, Gautambudh Nagar, Ghaziabad and Saharanpur. After laboratory confirmation out of 109 processed samples, only 41 were positive for E. coli. Out of these 41 isolates only 13 revealed the toxin production, hence were pathogenic. On the basis of drug sensitivity test, Amikacin (87.80% sensitivity), Aztreonam (73.17%) and Gentamicin (51.21%) were found to be the effective drugs which could prohibit the growth of E. coli isolates obtained during sudy. However, the presence of 100% resistance against Ampicillin, Cefdinir, Co–trimoxazole, Cloxacillin, Erythyromycin, Lincomycin, Penicillin, Rifampin, Tetracyclin and Vancomycin is matter of concern particularly the resistance against Norfloxacin and Pefloxacin as fluroquinoles are very commonly used in cases of gastroenteritis.
INTRODUCTION
Diarrhoea is one of the most common and multifactorial disease of neonates chiefly caused by E. coli and remains common devastating disease all over the world (Bhalerao et al., 2000; Kumar et al., 2013), particularly in calves less than three months of age (Malik et al., 2012 and 2013). The etiological agent E. coli is also considered as important food borne pathogen (Dhama et al., 2013a). Similarly, Sizov et al. (1984) also observed the presence of E. coli in 34 percent samples of calves in the age group of 3 to 10 days that died due to diarrhoea. Among the multiple predisposing factors of diarrhoea due to bacterial causes especially E. coli, risk is greater in calves in which there has been failure of passive transfer of maternal immunoglobulin (Gay 1983). Inspite of the complex etiology of calf diarrhea, the involvement of bacterial pathogens is still responsible for more than 50% cases of diarrhoea in neonatal calves (Kumar et al., 2012a,2012b) and E. coli is more or less consistently isolated during cultural examination of the intestinal contents of calves that succumb to diarrhoea complex during first three weeks of life (Boyd et al., 1974; Malik et al., 2012). Colibacillosis is one of the major causes of neonatal calf diarrhoea and it accounts for maximum neonatal calf mortality (Sherwood et al., 1983, Haggard, 1985, Mailk et al., 2012). Now Escherichia coli is seen as a pathogenic species with remarkable versatility in its ability to cause disease in both humans as well as animals. Depending upon the severity of condition and also availability of trend personnel antimicrobials are commonly used in therapy of diseased calves either orally or parentally. Some commonly used antimicrobials as sulbactam, ampicillin, neomycin, cephalosporin, tetracycline and sulphonamide– trimethiprim mixture all have all been used but day to day development of drug resistance problem envisages regular drug sensitivity results with the timely available antimicrobials in the market (Kumar et al., 2010). Similar to other part of world in India also microbiological work has been amply conducted for finding the association of different strains of E. coli with diarrhoea in calves by Sharma (1986), Chakraborty and Nag (1997) and Hussain and Saikia (2000). However, in last decade or so the development of drug resistance and the presence of multi drug resistant pathogenic bacteria are major problem in treating bacterial diseases (Kumar et al., 2012c; Anita et al., 2013; Dhama et al., 2013b). Use of antimicrobial in early stages of disease has been always improves survival rate and highly favored but it requires proper selection of drug in the given population (DuPont and Ericsson, 1993). Thus looking into the intricacy of calf diarrhoea due to E. coli, the present study was planned to assess incidence rate of E. coli in calf diarrhoea and antimicrobial drug sensitivity pattern using commonly available antibiotics in the treatment.
MATERIALS AND METHODS
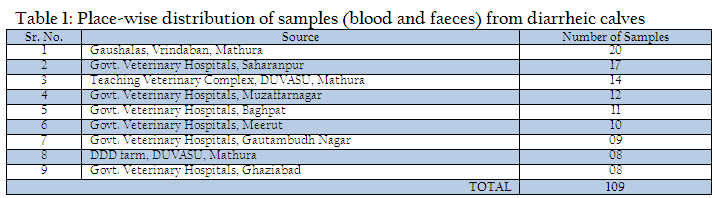
The present study was undertaken on calves up to three months of age exhibiting symptoms of diarrhea at teaching veterinary clinical complex, Instructional Livestock Farm Complex (DUVASU, Mathura), Nearby Gaushalas located at Vrindavan, Mathura along with some Veterinary Hospitals located in district Muzaffarnagar, Baghpat, Meerut, Gautambudh Nagar, Ghaziabad and Saharanpur of Uttar Pradesh state, India (Table 1). During the period under study, a total of 109 faecal samples from diarrhoeic calves were collected from the calves showing diarrhea and brought to laboratory on ice.
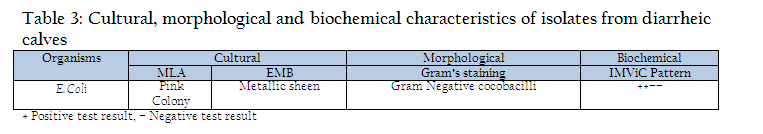
The faecal samples of diarrheic calves of less than 3 months of age were collected directly from rectum. Approximately 20 gms of faecal content were collected from rectum of calves in sterilized containers (Edwards and Ewing, 1972). The faecal samples were processed for isolation of Escherichia coli, as per standard procedure described by Edwards and Ewing (1972) for the characterization of isolates on the basis of routine laboratory procedures which included cultural, morphological and biochemical parameters by growth on differential media MLA (MacConkey’s lactose agar), selective media EMB (Eosin Methylene blue agar) of HiMedia (Mumbai), differential staining (Gram’s staining) and confirmed with biochemical test as Indole production, Methyl red reduction, Vogous Proskaure test and citrate utilization (IMViC) pattern (Quin et al., 2002).
All the isolates were examined for their drug sensitivity pattern by disc diffusion method (Bauer et al., 1966). The present study included 20 commonly used antibiotic discs (Hi–Media, Mumbai) viz., amikacin (30 mcg), ampicillin (10 mcg), aztreonam (30 mcg), cefadroxil (30 mcg), Cefdinir (5 mcg), ciprofloxacin (30 mcg), co–trimoxazole (25 mcg), cloxacillin (5 mcg), erythyromycin (15 mcg), gentamicin (10 mcg), kanamycin (30 mcg), lincomycin (15 mcg), norfloxacin (10 mcg), nitrofurantoin (300 mcg), pefloxacin (5 mcg), penicillin (10 IU), rifampin (5 mcg), tetracyclin (30 mcg), tobramycin (10 mcg), vancomycin (30 mcg). For the preparation of bacterial lawn on plates, six hours young broth culture (4.8x1010 c.f.u/ml) of each isolate was smeared over the nutrient agar medium by sterilized cotton swab. After the inoculation plates were allowed to dry at room temperature for 10–15 minutes. Then respective antibiotic discs were placed on the surface of inoculated medium by sterile forceps with uniform spacing between two discs and pressed gently to ensure full contact. For the final interpretation all the inoculated plates were incubated at 370C for overnight (Kumar et al., 2012c). After the incubation of 24 hrs, zone of inhibition was recorded in millimeters and compared with the chart provided by the manufacturer (HiMedia, Mumbai) for assessing the sensitivity of the antibiotics. The interpretation of results was performed as per the guidelines of NCCLS(2002)
Isolates of E. coli obtained in the study were tested for haemolysin production on 5 % sheep blood agar according to Beutin et al., 1989. For the confirmation of haemolysin production streaked blood agar plates with E. coli were incubated at 370C and examined at 4 hrs interval up to 24 hrs for the presence of zone of haemolysis.
RRESULTS
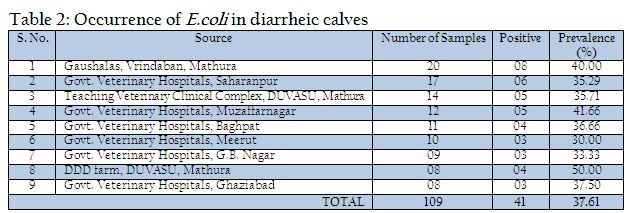
Out of 109 faecal samples, 47 samples produced smooth, circular, convex, entire, pink colour colonies on MLA. Further isolated pink colonies from MLA were transferred on selective media EMB plates. After overnight incubation at 370C, out of 47 samples only 41 produced metallic sheen on EMB plates (Table 2). The presumptive E. coli colonies were subjected to Gram’s staining and biochemical tests (Table 3). Thus, out of 109 faecal samples from diarrheic calves, E. coli could be confirmed from only 41 samples. Thus percent positivity of E. coli in diarrheic calves was 37.61% (Table 2).
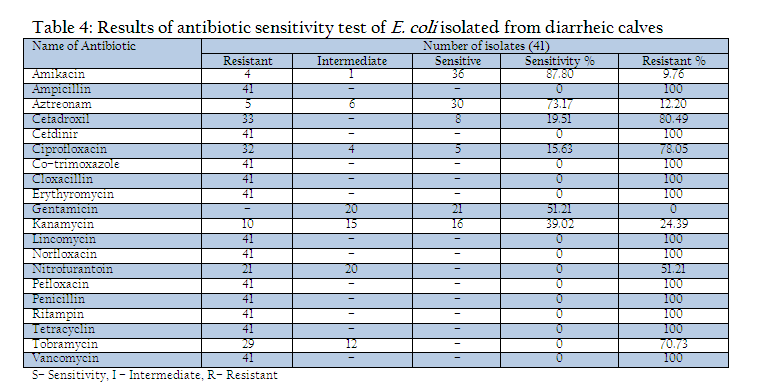
All the isolates were examined for their drug sensitivity pattern by disc diffusion method (Bauer et al., 1966) with selected antimicrobial discs (Hi–Media, Mumbai) and results of antibiogram revealed the presence of drug resistance in E. coli isolates recovered from diarrheic calves against most of the commonly used antibiotics such as ampicillin, Cefdinir, cotrimoxazole, cloxacillin, erythyromycin, lincomycin, norfloxacin, pefloxacin, penicillin, rifampin, tetracycline and vancomycin. Out of 20 drugs only three drugs mainly Amikacin (87.80% sensitivity), Aztreonam (73.17%) and Gentamicin (51.21%) were found to be the effective drugs which could prohibit the growth above 50% of isolates during the drug sensitivity test. The antibiotic discs of Kanamycin could showed only 39.0% sensitivity (Table 4).
In an effort to test the pathogenecity of isolates, all the 41 isolates of E. coli were tested for haemolysin production on sheep blood agar (SBA) as per Beutin et al. (1989). Haemolysin producing isolates of E. coli produced the typical hemolytic reaction after 4 hrs incubation on SBA whereas, after 24 hrs of incubation clear hemolysis was produced by 13 isolates. Isolates which produced no zone of haemolysis even after 24 hrs incubation on SBA were considered as non–hemolytic.
DISCUSSION
In the present investigation a total of 109 faecal samples were collected from diarrheic calves (below three months of age), for isolation and confirmation of E.coli. Out of 109 faecal samples of diarrhoeic calves, 41 faecal samples were positive for E. coli. The prevalence of E. coli in diarrhoeic calves was 37.61%. Yadav and Gupta (1971) noticed 35% prevalence of E. coli in diarrhoeic calves. Srisuparbh (1978) reported 26.25% prevalence of E. coli in diarrhoeic calves. Shome and Shome (1996) reported 14 samples positive for E. coli out of 25 faecal samples of diarrhoeic calves. Navade et al. (2000) noticed 53.37% prevalence of E. coli in neonatal diarrhoeic calves. Gupta et al. (2006) observed only 23.72% prevalence rate of E. coli in diarrhoeic calves. The findings of these workers are more or less similar to the findings of present study. The prevalence rate of E. coli in diarrheic calves varies in the range of 30% to 50%. The incidence of E. coli showed the higher prevalence at university dairy farm, Gaushalas in and around Vrindavan and Mathura. The higher prevalence of E. coli in these areas may be due to poor managemental practices and predisposing factors like overcrowding and malnutrition, which are supposed to be primary cause of immunosupression. As E. coli is a commensal organism and is responsible for diarrhea in majority of weak, malnourished, debilitated and immunosuppressed calves particularly calves receiving less or no maternal antibodies through colostrums (Malik et al., 2012). Comparatively lower prevalence rate of E. coli in diarrheic cases of district Meerut and Gautambudh Nagar as compared to Muzaffarnagar and Ghaziabad may be due to better managemental practices, individual rearing and better awareness among livestock owners and trend of feeding calves for colostrums. Major reason behind the practice in western Uttar Pradesh is that still individual livestock owners hesitate to sell the milk and main purpose of rearing these animals is production of milk for their own household consumption and also the breeding of animals for agriculture requirement. The resistance of all the E. coli isolates procured during the study period against commonly used antibiotics such as ampicillin, Cefdinir, co–trimoxazole, cloxacillin, erythyromycin, lincomycin, norfloxacin, pefloxacin, penicillin, rifampin, tetracycline and vancomycin is a matter of serious concern as Norfloxacin, pefloxacin and tetracycline are vary commonly used and prescribed antibiotics in veterinary and human practices even today. This resistance might be due to indiscriminate use of these antibiotics irrespective of etiological agents (Verma et al., 2007; Kumar et al., 2010) as well different drug interaction due to concurrent administration is performed irrespective of type of drug and their interaction (Rahal et al., 2007 and 2013). The findings of study are in contrast to the findings of Tripathi and Soni (1982), who observed 69.69% E. coli sensitive for ampicillin and 69.70% to Chloramphenicol. Bali et al, (2000) similar to our findings reported pefloxacin as an effective drug in enteric colibacillosis in calves and Chowdhury and Das (2003) found a high degree sensitivity of E. coli isolates to Ciprofloxacin, however, in present study the recovered isolates from the cases of diarrheic calves revealed 100% resistance against fluroquinolones group of antibiotics as norfloxacin and pefloxacin. The resistance against the antibiotics used for the treatment of disease conditions caused by gram positive bacteria as penicillin and tetracycline is supported by the findings of Tripathi and Soni (1982), who observed that 45% of E. coli showed resistance to Streptomycin, (75.75%) to oxytetracycline. Whereas Boyd et al, (1974) obtained 100% resistance against Penicillin G. In an another study similar to the findings of present study, Fairbrother et al., (1978) also observed 100% resistance with erythromycin, penicillin and resistance up to 95% against tetracycline. Similarly Awasthi and Rao (1980) recorded 90–95% resistance against penicillin and oxytetracyclin. The transfer of resistance factor from resistant microorganisms through R or RTF might be the cause of this high rate of resistance in E. coli isolates (Yeoman, 1980) and the other probable reason might be due to under dosing and irrational therapy particularly for duration of treatment (Kumar et al., 2012a, 2012b,2012c). In the present study, isolates of E. coli isolated from the cases of diarrhea in calves showed that 87.80% were sensitive to amikacin, 73.17% to aztreonam and 51.21% to gentamicin. Chowdhary and Das, 2003 and Sato et al., 2005 have already reported high rate of sensitivity against gentamicin. The hemolytic property of E. coli strain associated with diarrhea were recognized long back as early as by Dudgeon et al. (1921). This hemolytic activity is due to presence of toxin haemolysin and play important role in virulence of E. coli (Smith and Longgod, 1972). Hemolytic activities have been shown to be toxic to a wide range of mammidium cells including sheep erythrocytes (O’Hanley et al., 1991). ETEC and VTEC strains of E. coli produce alpha hemolysin and entrohemolysin, respectively, which are responsible for pathogenecity. Enterohemolysin is produced by VTEC but rarely by non–VTEC E. coli strains, which possess alpha hemolysins show typical clear zone of hemolytic reaction on sheep blood agar after 24 hr of incubation. Strains having entrohemolysin did not exhibit hemolytic reaction. During the study to assess the presence of haemolytic activity, thirteen isolates which revealed clear zone of hemolysis on SBA after 24hr of incubation were alpha hemolysin producing VTEC strains. The prevalence rate of these is 31.70% in the occurrence of E. coli. Thus the present study revealed 11.92% overall prevalence rate of alpha hemolytic VTEC strains.
The above findings also suggest that there is a constant need of screening of faecal samples of diarrheic calves this is especially important to formulate a suitable broad spectrum and effective treatment against the E. coli and to reduce or prevent the losses.
ACKNOWLEDGEMENTS
The authors are highly thankful to Dean, College of Veterinary Sciences and Animal Husbandry and Hon’ble Vice Chancellor, DUVASU, Mathura for providing all the facilities to conduct the study. Authors are also thankful to the staff of TVCC and other veterinary hospitals, Department of Veterinary Microbiology and Immunology, Department of Veterinary Epidemiology and Preventive Medicine and the animal owners, who allow their animals to take part in the study.
REFERENCES
Anita, Kumar A, Verma AK, Gupta MK and Rahal A (2013). Multi drug resistant pathogenic Escherichia coli in water sources and Yamuna river in and around Mathura, India. Pak. J. Biol. Sci.. DOI 10.3923/pjbs/2013.
Awasthi BK and Rao KNP (1980). Use of hyper immune serum for the treatment of colisepticemia in calves. Ind. J. Anim. Sci. 5: 44-45.
Bali RM, Mode SG, Kolte AY, Sadekar RD and Horne SD (2000). Efficacy of pefloxacin in entric colibacillosis in calves. Ind. Vet. J., 77 (11): 981-983.
Bauer AW, Kirb, WMM, Sherris JC and Turck M (1966). Antibiotic sensitivity testing by a standardized single disk method. Am. J. Clin. Path. 45:493-496
PMid:5325707
Beutin LMA, Montenegro I, Orskov F, Orskov J, Prada S, Zimmerman N and Stephan R (1989). Close association of verotoxin production with entrohemolysin production in strains of E. coli. J. Clin. Microbial. 27: 2559-64.
PMid:2681256
Bhalerao DP, Navade RN, Jagdish S, Samad A and Keskar DV (2000). Neonatal calf diarrhoea- therapeutic approach. Ind. Vet. J., 77(9): 817-818.
Boyd JW, Baker JR and Leyland A (1974). Neonatal diarrhoea in calves. Vet. Rec., 95: 310-313.
http://dx.doi.org/10.1136/vr.95.14.310
PMid:4615425
Chakarborty D and Nag NC (1997). Isolation and characterization of Escherichia coli associated with diarrheic cases of cattle and man. Ind. J. Ani. Health. 36:147-150.
Chowdhury M and Das R (2003). Incidence of drug resistance in E. coli strain in West Bengal. Ind.Vet. J., 80(1): 81-82.
Dhama K, Rajagunalan S, Chakraborty S, Verma AK, Kumar A and Tiwari R and Kapoor S (2013a). Food-borne pathogens of Animal origin-Diagnosis, prevention and control and their zoonotic significance- A review. Pak. J. Biol. Sci. 16(20): 1076-1085.
http://dx.doi.org/10.3923/pjbs.2013.1076.1085
PMid:24506006
Dhama K, Chakraborty S, Mahima, Wani MY, Verma AK, Deb R, Tiwari R and Kapoor S (2013b). Novel and emerging therapies safeguarding health of humans and their companion animals: A review. Pak. J. Biol. Sci. 16(3): 101-111.
http://dx.doi.org/10.3923/pjbs.2013.101.111
PMid:24171271
Dudgeon LS, Wordley E and Bawtrea G (1921). On bacillus cli infections of urinary tract especially in relation to hemolytic organism. J. hyg. 20: 137-164.
http://dx.doi.org/10.1017/S002217240003391X
DuPont HL and Ericsson CD (1993). Prevention and treatment of traveler's diarrhoea. N. Engl. J. Med. 328:1821-1827.
http://dx.doi.org/10.1056/NEJM199306243282507
PMid:8502272
Edwards R and Ewing WH (1972). Identification of Enterobacterceae. 3rd edn., Burgess Publishing Company, Minnesota. pp 337-356.
Fairbrother JM, McDonough PL, Shin SG and Acres SG (1978). A survey of drug resistance in E. coli isolated from neonatal calves in New York State from 1976 to 1978. Proceeding of second International Symposium on neonatal diarrhoea. Oct.3-5, 1978, National Informatics Centre, Indore, M. P.
Gay CC (1983). Failure of passive transfer of colostral immunoglobulins and neonatal diseases in calves: a review. VIDO in proceedings fourth Int. Symp. Neonatal diarrhoea, 346-364.
Gupta DK, Shukla SK, Rajora VS and Nag LK (2006). Prevalence of E. coli in the neonatal diarrhoeic calves. Ind. J. Vet. Med. 26(1): 36-37.
Haggard DL (1985). Bovine enteric colibacillosis. Vet. Clin. North Am. Food Anim. Prac., 1: 495-508.
PMid:3907783
Hussain and Saikia GK (2000). Isolation and characterization of bacteria from diarrhoeic bovine calves. Indian J. Comp. Microbiol. Infect. Dis. 21:125-127.
Kumar A, Rahal A, Dwivedi SK, Gupta MK (2010). Prevalence and antibiotic profile of bacterial isolates from bovine mastitis in Mathura. Egyptian J. of Dairy Sci. 38(1):31-34.
Kumar R, Verma AK, Kumar A, Srivastava M and Lal H P (2012a). Prevalence and antibiogram of campylobacter infections in dogs of Mathura, India. Asian J. Anim. Vet. Adv., 7(5): 734-740.
http://dx.doi.org/10.3923/ajava.2012.434.440
Kumar R, Verma AK, Kumar A, Srivastava M and Lal HP (2012b). Prevalence of campylobacter spp. in dogs attending veterinary practices at Mathura, India and risk indicators associated with shedding. Asian J. of Anim. Vet. Adv., 7(8):754-760.
http://dx.doi.org/10.3923/ajava.2012.754.760
Kumar A, Verma AK, Gangwar N and Rahal A (2012c). Isolaion, characterization and antibiogram of Mycoplasma bovis in sheep pneumonia. Asian J. Anim. Vet. Adv., 7(2): 149-157.
http://dx.doi.org/10.3923/ajava.2012.149.157
Kumar A, Verma AK, Malik S, Gupta MK, Sharma A and Rahal A (2013). Occurrence of extended spectrum Beta-lactamases producing alpha hemolytic Escherichia coli in Neonatal diarrhea. Pak. J. Biol. Sci. DOI 10.3923/pjbs/2013.
Malik S, Verma AK, Kumar A, Gupta MK and Sharma SD (2012). Incidence of calf diarrhea in cattle and buffalo calves in Uttar Pradesh, India. Asian J. Anim. Vet. Adv., 7: 1049-1054.
http://dx.doi.org/10.3923/ajava.2012.1049.1054
Malik S, Kumar A, Verma AK, Gupta MK, Sharma SD, Sharma AK and Rahal A (2013). Haematological profile and blood chemistry in diarrhoeic calves affected with colibacillosis. Journal of Animal Health and Production, 1(1): 10-14.
Navade RB, Bhalerao DP, Jagdish S, Samad A and Keskar DV (2000). Neonatal calf diarrhoea- Serotyping of E. coli isolates. Ind. Vet. J., 77 (9): 815-816.
NCCLS (2002). Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animal. Approved Standard, 2nd edn, (NCCLS, Wayne, PA), M 31-A2.
O'Hanley P, Lalonde G and Ji G (1991). Alpha hemolysin contributes to the pathogenecity of piliated diagalactoside binding E. coli in the kidney; efficacy of an alpha hemolysin vaccine in preventing renal injury in the BALB/C mouse model of pylonephritis. Infec. Imm., 59: 1153-1161.
PMid:1671776 PMCid:PMC258381
Quinn PJ, Markey BK, Carter ME, Donnelly WJC, Leonard FC (2002). Veterinary Microbiology and Bacterial Disease. Black Well Science, London. pp. 1-648.
Rahal A, Kumar A, Ahmad AH and Malik JK (2007). Pharmacokinetics of diclofenac and its interaction with enrofloxacin in sheep. Res. in Vety. Sci., 84: 452–456.
Rahal A, Ahmad AH, Kumar A, Mahima, Verma AK, Chakraborty S and Dhama K (2013). Clinical drug interaction: a holistic view. Pak. J. Biol. Sci. 16(16): 751-758.
http://dx.doi.org/10.3923/pjbs.2013.751.758
PMid:24498827
Sato K, Bartlett PC and Saeed MA (2005). Antimicrobial susceptibility of Escherichia coli isolated from dairy farms using organic versus conventional production methods. J. Am. Vet. Med. Assoc. 226(4): 589-594.
http://dx.doi.org/10.2460/javma.2005.226.589
PMid:15742702
Sharma M (1986). Studies on the efficiency of K99 E.coli bacterin in neonatal diarrhoea and seroprevalence of Rota virus in calves. M.V.Sc. submitted to CSAUAT, Kanpur.
Sherwood D, Snodgrass DR and Lawson GHK (1983). Prevalence of enterotoxigenic E.coli in calves. Vet. Record, 113: 208.
http://dx.doi.org/10.1136/vr.113.10.208
PMid:6356571
Shome R and Shome BR (1996). Bacteriological study on calf diarrhoea cases in Andamans. Ind. Vet. J., 73(9): 1001-1002.
Sizov I, Fetisova K, Nikolov ND, Georgiev GK and Ivanvov IT (1984). Virological and Bacteriological Investigation of gastroenteritis in new born calves. Vet. Med. Nauki. 21:89-94. PMid:6099625
Smith HW and Linggod MA (1972). Observation on pathogenic properties of K-88, Hly and Ent plasmids of E. coli with particular reference to porcine diarrhea. J. Med. Microbial. 4: 4467-4485.
Srisuparbh K (1978). Infection cause and diagnosis method in neonatal calf diarrhoea. Dissertation abstracts International., 39: 2032 (Vet. Bull., 1978, 50; Abst. 6067).
Tripathi RD and Soni JL (1982). Antibiotic sensitivity test with E. coli Isolates from cases of neonatal calf diarrhoea. Ind. Vet. J., 59: 413-456.
Verma AK, Sinha DK and Singh BR (2007). Salmonella in apparently healthy dogs. Journal of Veterinary Public Health. 5(1):37-39.
Yadav, JNS and Gupta, BM (1971). Antibiotics sensitivity pattern of strain of E.coli isolated from gastroenteritis of domestic animals. Ind. J. Pathl. Bacteriol. 14: 32.
Yeoman GH (1980). Antibiotics resistance in relation to neonatal diarrhoea of calves and piglets in Recent Advances in neonatal diarrhoea in farm animals: Symposium, University of Illinois, USA, pp 29-36.