Journal of Animal Health and Production
Research Article
Journal of Animal Health and Production 2 (2): 19 – 24Gill Disease Caused by Thelohanellus bifurcata a Pathogenic Myxozoan Parasite in Cultured Indian Carp, Labeo rohita (Hamilton, 1822) in Punjab, India
Harpreet Kaur**, Anu Katoch
*Corresponding author: harpreetbimbra@gmail.com
ARTICLE CITATION:
Kaur H, Katoch A (2014). Gill disease caused by Thelohanellus bifurcata basu and haldar, 1999 a pathogenic myxozoan parasite in cultured Indian carp, Labeo rohita (Hamilton, 1822) in Punjab, India. J Anim. Health Prod. 2 (2): 19 – 24
Received: 2014–05–28, Revised: 2014–06–17, Accepted: 2014–06–18
The electronic version of this article is the complete one and can be found online at
(http://dx.doi.org/10.14737/journal.jahp/2014/2.2.19.24)
which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Abstract
A myxozoan parasite, Thelohanellus bifurcata Basu and Haldar, 1999 infecting gills of cultured major carp, Labeo rohita (Hamilton, 1822), rohu was collected from 4 fish farms in Patiala district (Punjab), India. The infection rate was 20% (3/15) and the age of the fishes examined was between 3–10 months. A total of 20–30% fish mortality was recorded which included young fish between the age of 3–4 months. Plasmodia were large, visible with naked eye, oval and creamish white in color enclosing 1000–1200 spores. Spores histozoic, large, elongate pyriform in shape, tapering anteriorly with a notch–like constriction at 3/4th of the spore body and were characterized in having anteriorly bifurcated polar capsule. In histopathological sections each plasmodium occupied almost 15–25% of the gill filament. The cavity of the plasmodium was filled with spores and cellular debris. The pathogenesis caused by T. bifurcata was evident in cultured carps as infection caused severe damage to the gills leading to total loss of its respiratory surface. According to their location on the gill filament the plasmodia were of two types i.e. intrafilamental vascular type (FV2) occupying the tip portion and intralamellar vascular type (LV3) occupying side of the gill filament.
INTRODUCTION
Parasites and diseases are the serious factors in determining the success of aquaculture practices. In Punjab, fishes are generally cultured in large number in a limited area. In intensive and polyculture system, the fish pathogens can easily be transmitted through water and mud. In culture ponds, the myxosporidian infections are the most common causes of mortality thereby affecting fish production drastically. The pathological symptoms caused by myxozoan parasites have been studied by many workers such as Chakravarty (1939), Mitchell (1977), Kalavati and Narasimhamurti (1985), Awal et al., (2001), Molnar (2002), Basu and Haldar (2002, 2004), Adriano et al., (2009), Chavda et al., (2010), Campos et al., (2011), Raissy and Ansari (2011) and Kaur et al., (2013). In aquaculture, all the three major carps namely, Labeo rohita Hamilton, Cirrhinus mrigala Hamilton and Catla catla Hamilton have been found to be infected with myxozoan parasites (Azevedo et al., 2010; Hemananda et al., 2009, 2012; Manrique et al., 2012). Gill and dermal lesions due to myxozoan infection have been reported in many states of India (Chakravarty, 1939; Gupta and Khera, 1989; Padma Dorthy and Kalavati, 1992; Kalavati and Vaidehi, 1992; Mohan and Shanker, 1995; Kalavati and Nandi, 2007; Dar, 2013; Kaur et al., 2013). Besides this, many species of myxosporean parasites were recorded from freshwater fishes of wetlands of Punjab (Kaur and Singh, 2008, 2009, 2010a, 2010b, 2011a, 2011b, 2011c, 2011d, 2011e, 2011f, 2012a, 2012b; Singh, 2011). In Punjab, the aquaculture is an upcoming practice being taken up by many farmers as the natural stock declined and representing it as the fastest growing animal husbandry. Presently, the area under fish culture is 10856.6ha and number of farms are 8285. However, poor knowledge of the prevailing disease causing organisms and lack of proper management practices have led to the harmful effects on fish production (Dhawan, 2006). Among myxosporidians, the two genera, Myxobolus and Thelohanellus have been recorded as the most common in aquaculture (Sarkar and Raychaudhuri, 1986; Adriano et al., 2009). In the present study, T. bifurcata Basu and Haldar, 1999 has been recorded for the first time from a fish pond in Punjab (earlier was reported from Nanaghat, District Nadia, West Bengal). In the present study, T. bifurcata Basu and Haldar, 1999 has been redescribed and its histopathology on the gills have been investigated in detail.
MATERIALS AND METHODS
Sample Origin
Fishes from 4 fish farms– Kuku Dala, Dhindsa, Nanoki and a Government fish farm located in district Patiala, Punjab were procured in live condition and brought to the laboratory for further investigation. Fishes ranged 25–30cm in length and were 8–10 months old. The selected ponds were polyculture having Labeo rohita Hamilton, Catla calta Hamilton, Cirrhinus mrigala Hamilton, Ctenopharyngodon idellus Valenciennes and Cyprinus carpio Linnaeus. The temperature of the pond water at the time of the collection was 30–32˚C. The organs such as gills, gut, eyes, fins, scales and skin were examined for infection. Infected organs were fixed in Bouin’s fixative for tissue location and histopathological studies.
Preparations of Slides
Fresh Spores (Temporary Preparations)
Each plasmodium was ruptured in normal saline (0.85%) with the help of a needle on a clean slide and examined under the light microscope for the presence of spores. Fresh spores were studied in Lugol’s iodine solution to confirm the presence of iodinophilous vacuole. Polar filament was extruded by treating the spores with 8% KOH.
Staining of Spores (Permanent Preparations
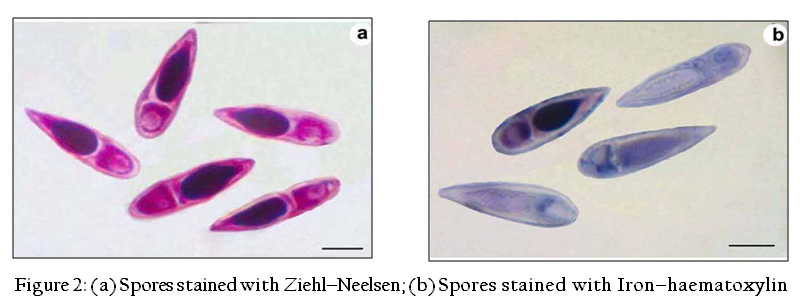
- Fixation: To make dry preparations thin smears were air dried, fixed in methanol and stained with Giemsa. In the case of permanent (wet) preparations, smears on clean slides were fixed in Schaudinn’s and Bouin’s fixatives
- Staining: The stains such as Heidenhain’s Iron–haematoxylin and modified Ziehl–Neelsen were used to study the detailed spore morphology (Figure 2a, b)
Histopathology
Infected gills were cut into small pieces and fixed in Bouin’s fixative. Tissue samples were dehydrated in ascending grades of ethanol, cleared in xylene, embedded in paraffin wax, sectioned at 3–4µm thickness and stained with haematoxylin + eosin and Luna’s staining method (Luna, 1968). In Luna’s method, the cysts stained bright red and rest of the gill tissue stained blue in color. This technique demonstrated the location of cysts within the gills and therefore was considered useful in histological diagnosis of the myxosporean parasites.
RESULTS AND DISCUSSION
Thelohanellus Bifurcata Basu and Haldar, 1999 (Tables: I, II)
Plasmodia
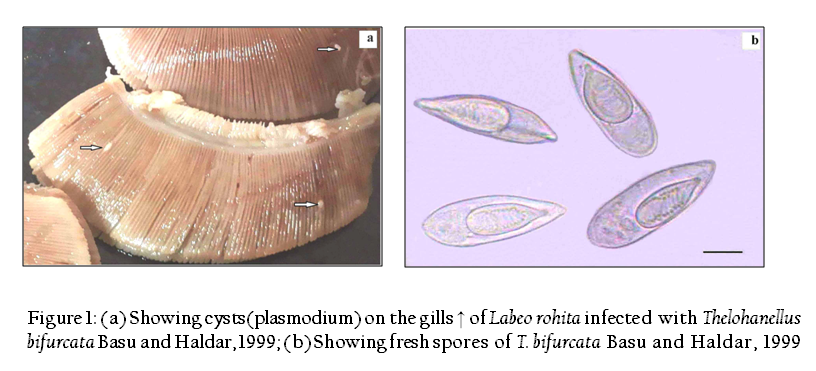
Large, creamish white, oval, present on the gill filament, 3–4 in number, measure 0.8–1.5x1.5–2mm in size, 1000–1200 spores per plasmodium (Figure 1).
Spore description (Table I)
(Measurements based on 7–8 spores in frontal view)
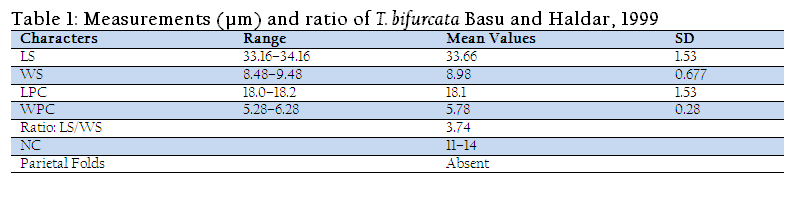
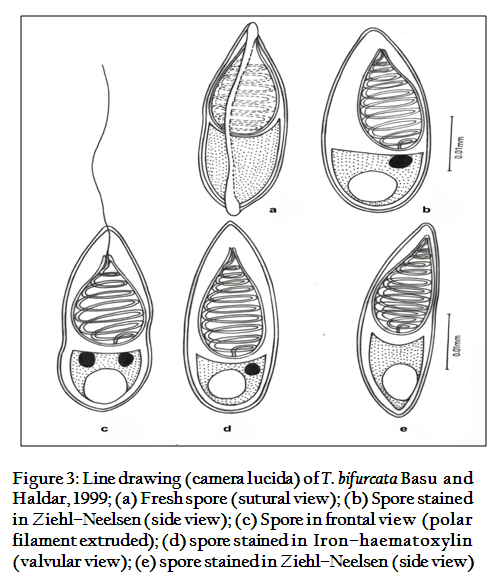
The spores are histozoic, large, measure 33.66x8.98µm, elongate pyriform in valvular view, tapering anteriorly with bluntly pointed anterior end and a notch–like constriction at 3/4th of the spore body, and broad rounded at the posterior end. Shell valves thick, smooth, symmetrical measuring 0.883µm in thickness with slightly curved and distinct sutural line. Parietal folds are absent. Polar capsule is pyriform in shape measure 18.1x5.78µm, with bifurcated anterior end and rounded posterior end. Polar capsule is situated anteriorly and occupies nearly half of the spore body cavity. Polar filament form 11–14 coils arranged perpendicular to the polar capsule axis and is thick, thread–like measuring 20µm in length when extruded. Sutural line is straight and distinct. Sporoplasm homogenous, granular occupies whole of the extracapsular space behind the polar capsule. An iodinophilous vacuole is present, measuring 4.78µm in diameter.
Taxonomic summary of T. bifurcata Basu and Haldar, 1999
Host :Labeo rohita (Ham.) vern. rohu
Locality :Cultured farm, Patiala (Punjab)
Site of infection :gill filament (intralamellar and intrafilamental)
Prevalence of infection :20% (3/15)
Pathogenicity :highly pathogenic
Fish mortality :20–30%
Remarks
The present observations (LS/WS: 3.74) on T. bifurcata Basu and Haldar, 1999 are in conformity with the original description (LS/WS: 3.78) (Table II), however, spore and polar capsule are smaller in size in the present species. T. bifurcata Basu and Haldar, 1999 has been recorded for the first time from Punjab and histopathology has been done for the first time. Earlier, T. bifurcata Basu and Haldar, 1999 was reported as non pathogenic in the gill filaments of a hybrid of Labeo rohita and Catla catla (Kalavati and Nandi, 2007). The present study indicated extensive damage to the respiratory surface of the gills due to large sized plasmodia of T. bifurcata Basu and Haldar, 1999 and hence proved to be highly pathogenic to the cultured fish.
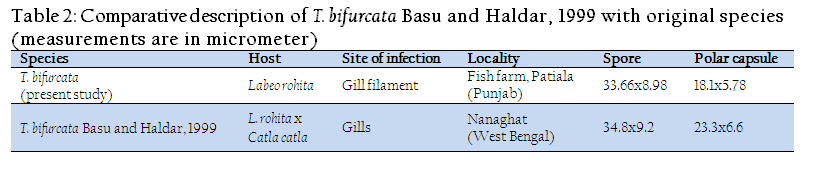
Table 2: Comparative description of T. bifurcata Basu and Haldar, 1999 with original species(measurements are in micrometer)
Histopathology
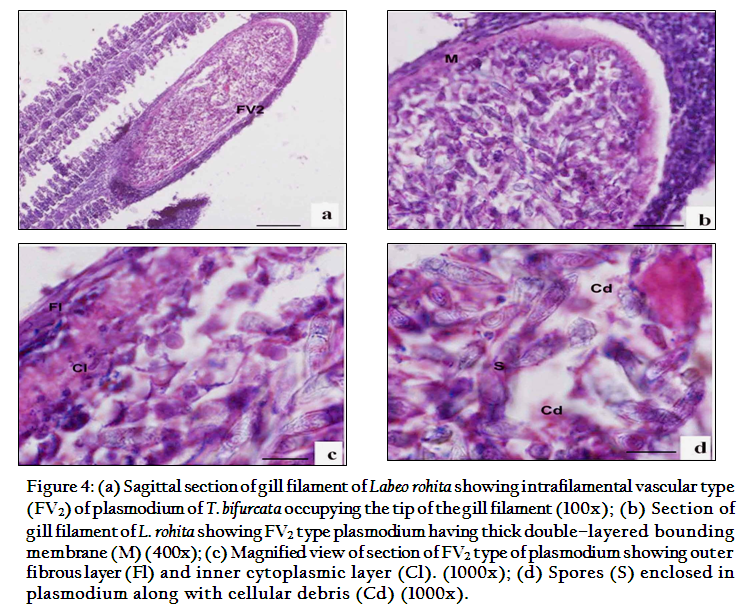
The present study indicated that T. bifurcata Basu and Haldar, 1999 as highly pathogenic parasite of gills of cultured Labeo rohita. The plasmodia were large, creamish white cysts visible with naked eye. 3–4 plasmodia in each gill towards the lower side of the arch were present (Figure 1a). Histopathological sections indicated the presence of cysts in two locations on the gills, one at the tip of the gill filament and other below the tip on one side of it. In the region of location of the cysts on the gill filament caused complete loss of respiratory surface. Both the plasmodia occupied up to 15–25% of the total surface area of the gill filament. Plasmodia ranged 0.8–1.5mm x 1.5–2mm in size each enclosing 1000–1200 large sized spores.
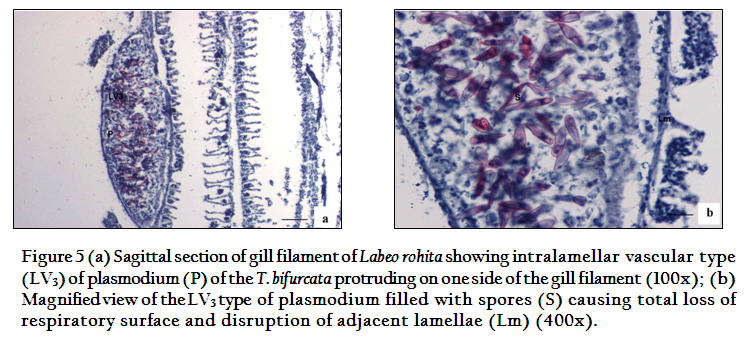
According to the location on the gill filament plasmodia were of two types and were classified as per Molnar (2002). The first type of plasmodium occupying the tip of the gill filament was intrafilamental vascular type (FV2). This was formed by the fusion of several plasmodia into a large, oval cyst, measuring 2.00x1.5mm in size located at the tip of the filament (Figure 4). The intrafilamental vascular (FV2) type of plasmodia of T. bifurcata occupied 15–20% of each gill filament. The size of the plasmodium exceeded the width of the gill filament. Several gill lamellae were fused and compressed resulting in reduction of the respiratory surface. The wall of the FV2 plasmodia was thick, made up of two layers, inner cytoplasmic measuring 0.0167mm and outer fibrous measuring 0.033mm in thickness. The lumen of the plasmodium was filled with spores and cellular debris. The second type of plasmodia were large, oval shaped measuring 1.6x0.8mm in size and were intralamellar vascular type (LV3), localized in vascular tissue of the gill filament. (Figure 5). This type of plasmodium occupied side of the gill filament (below the tip) and caused destruction of several gill lamellae and majority of them were no longer discernible. In contrast to FV2 type of plasmodium, the wall of the LV3 was single layered made up of a thin cytoplasmic layer measuring 6µm in thickness. The cytoplasm of plasmodia was filled with debris due to destruction of vascular and cartilaginous tissue of the gill filament. Damage to the gills resulted in reduction of respiratory surface leading to severe stress due to lack of oxygen supply.
During the present study, visible plasmodia were observed on the gill filament formed by T. bifurcata Basu and Haldar, occupying approximately 15–25% of the total filament, supply of blood is interrupted due to the formation of LV3 type of plasmodia within the gill filament. Adriano et al., (2009) made similar observations that the presence of plasmodia affect the gill functions and drastically reduces the respiratory surface. Sanaullah et al., 1980 and Dykova and Lom, 1988 also reported myxoboliasis caused by myxozoans in the gills of Catla catla and gible carp. According to them, infected carps exhibited hemorrhagic condition with necrotic changes in the epithelia and in the connective tissues of gills. Earlier, similar observation have also been made by MacCraren et al., (1975) in gill infections of American catfish with Henneguya exilis, Kalavati and Narasimhamuri (1985) in Channa punctata, with Henneguya waltairensis and Rukyani (1990) in carp with Myxobolus koi. According to Schulman (1957) and Kalavati and Narashimhamurti (1985) rupturing of cysts can also lead to hemorrhages, sometimes resulting in considerable loss of blood as also seen in the present study. Histopathological study of the gills showed number of cysts and parasites in the gills with a slight hyperplasia of the cells and epithelium. Severe infection caused hyperplasia of the basal epithelial and goblet cells leading to increase in mucus production. The intrafilamental as well as intralamellar location of cysts was recorded to be associated with hyperplasia and inflammation. These cellular changes led to the fusion of adjoining secondary lamellae. Respiratory distress syndrome and suffocation of fishes due to myxozoan infection have also been observed by various workers such as Dykova and Lom (1978); Current and Janovy (1978); Shariff (1982); Bowser and Conroy (1985); Kalavati and Narasimhamurti (1985) and Martins et al., (1997).Some authors have also described severe lesions caused by various species of Myxobolus in carp leading to gill necrosis and gut degeneration (Molnar and Kovacs–Gayer, 1985; Dykova and Lom, 1988). Barassa et al., (2003), Eiras et al., (2008, 2009) and Adriano et al., (2009) reported the histological analyses of gills in-fected with myxozoan parasites revealed presence of numerous large cysts in the gill filaments, but no pronounced inflammatory response was found in the infection site as also evident from the present study. The structural alterations in the gills are similar to structural changes reported for other myxosporean species (Feist and Longshaw, 2006). The wall of the plasmodia is double layered outer is fibrous and inner is cytoplasmic, similar observations were made by Casal et al., (2002, 2006) but differed from single layered plasmodia studied by Current et al., 1979. Gill infections by myxoporeans in farmed fish can cause significant tissue damage and occasionally death (Martins et al., 1999; Adriano et al., 2005a, 2005b; Feist and Longshaw, 2006).
ACKNOWLEDGEMENTS
The authors acknowledge for providing financial support by University Grants Commission (UGC) Govt. of India in the form of Major Research Project (MRP).
CONFLICT OF INTEREST
No conflict of interest.
REFERENCES
Adriano EA, Arana S, Alves AL, Silva MR, Ceccarelli PS, Henrique–Silva F, Maia AA (2005a) Histology, ultrastructure and prevalence of Henneguya piaractus (Myxosporea) infecting Prochilodus lineatus (Pisces: Prochilodontidae) cultivated in Brazil. Dis. Aquatic Org. 64: 229–235.
http://dx.doi.org/10.3354/dao064229
PMid:15997821
Adriano EA, Arana S, Alves AL, Silva MR, Ceccarelli PS, Henrique–Silva F, Maia A.A. (2005b). Histology, ultrastructure and prevalence of Henneguya caudalongula sp. n. (Myxosporea) infecting Prochilodus lineatus (Pisces: Prochilodontidae) cultivated in State of Sao Paulo, Brazil. Mem. Inst. Oswaldo Cruz. 100:177–181.
http://dx.doi.org/10.1590/S0074-02762005000200011
PMid:16021305
Adriano EA, Arana S, Alves AL, Silva MR, Ceccarelli PS, Henrique–Silva F, Maia AA (2009). Myxobolus cordeiroi n. sp., a parasite of Zungaro jahu (Siluriformes: Pimelodiade) from Brazilian Pan¬tanal: morphology, phylogeny and histopathology. Vet. Parasitol. 162: 221–229.
http://dx.doi.org/10.1016/j.vetpar.2009.03.030
PMid:19372007
Awal MA, Begum AA, Chandra KJ, Ahmed GU, Kurohmaru M (2001). Myxosporidian infection of gills and skin among carp from nursery ponds in Bangladesh: histopathology. Vet. Arhiv. 71(5): 265–276.
Azevedo C, Casal G, Mendonça I, Carvalho E, Matos P, Matos E (2010). Light and electron microscopy of Myxobolus sciades n. sp. (Myxozoa), a parasite of the gills of the Brazilian fish Sciades herzbergii (Block, 1794) (Teleostei: Ariidae). Mem. Inst. Oswaldo Cruz. 105(2): 203–207.
http://dx.doi.org/10.1590/S0074-02762010000200016
PMid:20428682
Barassa B, Adriano EA, Cordiero NS (2003). Henneguya curvata n. sp. (Myxosporea: Myxobolidae) parasitizing the gills of Serrasalmus spilopleura (Characidae : Serrasalminae), a south American fresh water fish. Folia Parasitol. 50: 151–153.
http://dx.doi.org/10.14411/fp.2003.026
Basu S, Haldar DP (1999). Thelohanellus bifurcata n. sp. a new species of the genus Thelohanellus from hybrid carps and checklist of the species of the genus described from Indian fishes. Proc. Zool. Soc. 52(1), 115–124.
Basu S, Haldar DP (2002). Myxobolous opthalmusculata, a new species of histozoic myxozoan (Myxozoa: Bivalvulida) from Cirrhinus mrigala (Hamilton). Proc. Zool. Soc. 55(2): 43–48.
Basu S, Haldar DP (2004). Description of three new species (Myxozoa: Myxosporea: Bivalvulida) of the genera Myxobilatus Davis, 1944 and Myxobolus Butschli, 1882. Acta Protozool. 43 (4): 337–343.
Bowser PR, Conroy JD (1985). Histopathology of gill lesions in channel catfish associated with Henneguya. J. Wildlife Dis. 21(2):177–179.
http://dx.doi.org/10.7589/0090-3558-21.2.177
PMid:3999252
Casal G, Matos E, Azevado C (2002). Ultrastructural data on the spore of Myxobolus maculates n. sp. (Phylum Myxozoa) parasite from American fish Metynnis argenteus (Teleostei). Dis. Aquatic Org. 51: 107–112.
http://dx.doi.org/10.3354/dao051107
PMid:12363082
Casal G, Matos E, Azevado C (2006). A new Myxozoan parasites from the Amazonian fish Metynnis argenteus (Teleostei, Characidae): Light and electron microscope observations. J. Parasit. 92: 817–821.
http://dx.doi.org/10.1645/GE-750R.1
PMid:16995400
Campos CM, Moraes JRE, Moraes FR (2011). Histopathology of gills of Piaractus mesopotamicus (Holmberg, 1887) and Prochilodus lineatus (Valenciennes, 1836) infested by monogenean and myxosporea, caught in Aquidauana river, State of Mato Grosso do Sul, Brazil. Rev. Bras. Parasitol.Vet. 20: 67–70.
http://dx.doi.org/10.1590/S1984-29612011000100014
PMid:21439236
Chakravarty MM (1939). Studies on myxosporidia from fishes of Bengal, with a note on myxosporidian infection in aquaria fishes. Arch. Protistenkunde. 92: 169–178.
Chavda D, Bhatt S, Sreepada RA, Sheth A (2010). Pathogenicity of Myxobolus infection and its effect on protein expression in Catla catla in central Gujarat region. J. Cell Tissue Res. 10(1): 2157–2164.
Current WJ, Janovy J (1978). Camparative study of ultrastructure of interlamellar and intralamellar types of Henneguya exilis Kudo from channel catfish. J. Protozool. 25: 56–65.
http://dx.doi.org/10.1111/j.1550-7408.1978.tb03868.x
PMid:660571
Current WJ, Janovy J (1979). Myxosoma funduli Kudo (Myxosporida) in Fundulus kansae: ultrastructure of plasmodium wall and of sporogenesis. J. Protozool. 26: 574–583.
http://dx.doi.org/10.1111/j.1550-7408.1979.tb04198.x
Dar SA (2013). Identification and distribution of myxozoan parasites infecting aquaculture fishes of district Patiala, Punjab. Dissertation. Punjabi University, Patiala (India)
Dhawan A (2006). Diversification from major carp farming–promising option in Punjab. Fish. Chimes. 25(10): 136–140.
Dykova I, Lom J (1978). Histopathological changes in fish gills infected with myxosporidian parasites of genus Henneguya. J. Fish Biol.12: 197–202.
http://dx.doi.org/10.1111/j.1095-8649.1978.tb04165.x
Dykova I, Lom J (1988) Review of pathogenic myxosporeans in intensive culture of carp (Cyprinus carpio) in Europe. Folia Parasitol. 36: 289–307.
Eiras JC, Takemoto RM, Pavanelli GC (2008). Henneguya caudicula n. sp. (Myxozoa, Myxobolidae) a parasite of Leporinus lacustris (Osteichthyes, Anostomidae) from high Parana river, Brazil, with revision of Henneguya spp infecting South American fish. Acta Protozool. 47: 149–154.
Eiras JC, Takemoto RM, Pavanelli GC (2009). Henneguya corruscans n. sp. (Myxozoa, Myxobolidae) a parasite of Pseudoplatystoma corruscans (Osteichthyes, Anostomidae) from Parana river, Brazil, a morphological and morphometric study. Vet. Parasitol. 159:154–158.
http://dx.doi.org/10.1016/j.vetpar.2008.10.020
PMid:19022582
Feist SW, Longshaw M (2006). In: Fish diseases and disorders. Protozoan and Metazoan Infections (ed. by P.T.K. Woo), pp. 230–296. CAB International, Oxfordshire.
Gupta S, Khera S (1989). Observations on Myxobolus punjabensis sp. nov. (Myxozoa:Myxobolidae),parasitic on gills and fins of Labeo dyocheilus.Riv.Parasitol.50:131–138.
Hemananda T, Mohilal N, Bandyopadhyay PK, Mitra AK (2009). Two new myxosporidia (Myxozoa: Myxosporea) of the genus Myxobolus Butschli, 1882 from cornea of Clarias batrachus (Linnaeus, 1758) caught from fish farm in India. N–W J. Zool 5(1), 165–169.
Hemananda T, Mohilal N, Bandyopadhyay PK, Mitra AK (2012) Myxobolus leafa sp. nov. (Myxozoa: Bivalbulida) from the gill filament of Labeo bata (Hamilton) from Manipur, India. Türkiye Parazitol. Derg. 37: 40–43.
Kalavati C, Nandi NC (2007). Handbook of Myxosporidean parasites of Indian fishes. ZSI, India, Kolkata.
Kalavati C, Narasimahamurti CC (1985). Histopathological changes in the gills of Channa punctatus BL. infected with Henneguya waltairensis. Arch. Protistenkunde. 129:199–202.
http://dx.doi.org/10.1016/S0003-9365(85)80023-3
Kalavati C, Vaidehi J (1992). A new myxosporidian, Thelohanellus chilkensis n. sp. from gills of the common carp, Labeo rohita in Chilka lake, India. Riv. Parassit. 33(57):79–82.
Kaur H, Katoch A, Dar SA, Singh R (2013). Myxobolus nanokiensis sp. nov. (Myxozoa: Bivalvulidae), a new pathogenic myxosporean parasite causing haemorrhagic gill disease in cultured Indian major carp fish, Labeo rohita (Hamilton 1822) in Punjab, India. J. Parasitic Dis. doi: 10.1007/s12639–013–0351–0.
http://dx.doi.org/10.1007/s12639-013-0351-0
Kaur H, Singh R (2008). Observations on one new species of genus Myxobolus – M. naini and rediscription of M. magauddi recorded from freshwater fishes of Kanjali Wetland of Punjab, India. Proceedings of 20th National Congress of Parasitology NEHU Shillong, India. pp. 75–79.
PMCid:PMC2822324
Kaur H, Singh R (2009). A new myxosporean species, Myxobolus eirasi sp. nov., a known species M. venkateshi Seenappa and Manohar (1981) from the Indian major carp fish Cirrhina mrigala (Ham). Protistology 6: 126–130.
Kaur H, Singh R (2010a). A new myxosporean species Myxobolus sclerii sp. nov. and one known species M. stomum Ali et al. (2003) from two Indian major carp fishes. J. Parasitic Dis. 34: 33–39.
http://dx.doi.org/10.1007/s12639-010-0010-7
PMid:21526031 PMCid:PMC3081699
Kaur H, Singh R (2010b). One new myxosporidian species, Myxobolus slendrii sp. nov., one known species, M. punjabensis Gupta, Khera (1989) infecting freshwater fishes in wetlands of Punjab, India. Parasitol. Res.106, 1043–1047.
http://dx.doi.org/10.1007/s00436-010-1746-9
PMid:20180134
Kaur H, Singh R (2011a) Two new species of Myxobolus (Myxozoa: Myxosporea: Bivalvulida) from freshwater fishes of Punjab wetlands (India). J. Parasitic Dis. 35: 33–41.
http://dx.doi.org/10.1007/s12639-011-0024-9
PMid:22654312 PMCid:PMC3114975
Kaur H, Singh R (2011b) Two new species of Myxobolus (Myxozoa: Myxosporea: Bivalvulida) infecting an Indian major carp in Ropar and Kanjali wetlands (Punjab). J. Parasitic Dis. 35(1) : 23–32.
http://dx.doi.org/10.1007/s12639-011-0033-8
PMid:22654311 PMCid:PMC3114976
Kaur H, Singh R (2011c) Two new species of Myxobolus (Myxozoa: Myxosporea: Bivalvulida) infecting an Indian major carp and a cat fish in wetlands of Punjab, India. J. Parasitic Dis. 35:169–176.
http://dx.doi.org/10.1007/s12639-011-0061-4
PMid:23024499 PMCid:PMC3235390
Kaur H, Singh R (2011d).Two new species of Myxobolus (Myxozoa: Myxosporea: Bivalvulida) infecting Indian freshwater fishes in Punjab wetlands (India). Parasitol. Res.108, 1075–1082.
http://dx.doi.org/10.1007/s00436-011-2307-6
PMid:21394535
Kaur H, Singh R (2011e). Myxobolus harikensis sp. nov. (Myxozoa: Myxobolidae) infecting fins of Cirrhina mrigala (Ham.), an Indian major carp in Harike wetland, Punjab (India). Parasitol. Res.109:1699–1705.
http://dx.doi.org/10.1007/s00436-011-2445-x
PMid:21584631
Kaur H, Singh R (2011f). Two new and one already known species of Myxobolus (Myxozoa: Myxosporea: Bivalvulida) infecting gill lamellae of Indian major carp fishes in Ropar and Harike wetlands (Punjab). Proceedings of 22nd National Congress of Parasitology, University of Kalyani West Bengal, India. pp. 81–90.
Kaur H, Singh R (2012a). A synopsis of the species of Myxobolus Bütschli, 1882 (Myxozoa: Bivalvulida) parasitizing Indian fishes and a revised dichotomous key to myxosporean genera. Syst. Parasitol. 81: 17–37.
http://dx.doi.org/10.1007/s11230-011-9321-z
PMid:22139007
Kaur H, Singh R (2012b). One new myxosporean species, Triangula cirrhini sp. n., and one known species, T. ludhianae (syn. M. ludhianae Gupta and Khera, 1991) comb. n. (Myxozoa: Myxosporea), infecting Indian major carp in Harike wetland of Punjab. Anim. Biol. 62: 129–139.
Luna LG (1968). Manual of histologic staining method of the Armed Forces Institute of Pathology, pp. 111.
MacCraren JP, Landolt MM, Hoffman GL (1975). Variation in response of Channel catfish to Henneguya sp. infections (Protozoa: Myxosporidia). J. Wildlife Dis. 11:2–7.
http://dx.doi.org/10.7589/0090-3558-11.1.2
Manrique WG, Claudiano GS, Figueiredo MAP, Petrillo TG, Moraes JRE, Moraes FR (2012). Myxosporidiosis in intensively–reared Piaractus mesopotamicus: Histopathological diagnosis by means of Ziehl–Neelsen staining. Pesq. Vet Bras. 32(11): 1133–1137.
http://dx.doi.org/10.1590/S0100-736X2012001100010
Martins ML, Souza VN, Moraes FR, Moraes JRF, Costa AJ, Rocha U F (1997). Pathology and behavioral effects associated with Henneguya sp. (Myxozoa: Myxobolidae) infections of captive Piarcactus mesopotamicus in Brazil. J. World Aq. Soc. 28: 297–300.
http://dx.doi.org/10.1111/j.1749-7345.1997.tb00646.x
Martins ML, Souza VN, Moraes FR, Moraes JRF Costa AJ, Rocha UF (1999). Gill infection of Leporinus macrocephalus Garavello & Britski, 1988 (Osteichthyyes: Anostomidae) by Henneguya leporinicola n. sp. (Myxozoa: Myxobolidae). Description, histopathology and treatment. Rev. Bras. de Parasit. Vet. 59(3): 527–534.
Mitchell LG (1977). Myxosporidia. In: Kreier JP (ed.) Parasitic Protozoa. Academic Press Inc New York. 4:115–154.
Mohan CV, Shankar KM (1995). Pathogenic myxosporean infection in early fry of Indian major carp, Catla catla. Curr. Sci. 68(5): 548–551.
Molnar K (2002). Site preference of fish myxosporeans in the gill. Dis. Aq. Org. 48: 197–207.
http://dx.doi.org/10.3354/dao048197
PMid:12033706
Molnar K, Kovács–Gayer E (1985). The pathogenicity and development within the host fish of Myxobolus cyprini Doflein. Parasitology, 90: 549–555.
http://dx.doi.org/10.1017/S0031182000055530
Padma Dorothy K, Kalavati (1992). Two myxosporean parasites of the mullet, Liza macrolepis (Smith). Acta Protozool. 32: 123–125.
Raissy M, Ansari M (2011). Histopathological changes in the gills of naturallyinfected Capoeta aculeate (Cuvier and Valenciennes, 1844) with parasites. African J. Biotech. 10(68): 15422–15425.
http://dx.doi.org/10.5897/AJB11.1838
Rukyani A (1990). Histopathological changes in gills of common carp (Cyprinus carpio L.) infected with the myxosporean parasite Myxobolus koi Kudo, 1920. Asian Fish. Sci. J. 3:337–341.
Sanaullah M, Ahmed ATA (1980). Gill myxoboliasis of major cars in Bangladesh. J. Fish Dis. 3: 349–354.
http://dx.doi.org/10.1111/j.1365-2761.1980.tb00404.x
Schulman SS (1957). The pathogenicity of the myxosporidian Myxobolus exiguous in correlation to its epizootics. Parasites and diseases of fish. Bull. Inst. Freshwater Fish. 42: 326–328.
Shariff N (1982). Henneguya shaharini sp. nov. (Protozoa: Myxozoa), a parasite of marble goby, Oxieleotrix marmoratus (Bleeker). J. Fish Dis. 5: 37–45.
http://dx.doi.org/10.1111/j.1365-2761.1982.tb00454.x
Sarkar NK, Raychaudhuri S (1986). Thelohanellus bengalensis sp. n. and Myxidium mystuium sp. n. (Myxozoa): Two new myxosporidia from Indian freshwater teleost. Acta Protozool. 25 (3): 359–362.
Singh R (2011). A study on the myxozoan parasites of the fishes of Punjab wetlands. Ph.D Thesis. Punjabi University, Patiala.