Journal of Animal Health and Production
Research Article
Ameliorative Effect of Vitamin E and Panax ginseng Against Some Adverse Effects of Levofloxacin in Male Rats
Hosny A. E. Ibrahim1, Nevertyty M. Mahmoud2, Dalia Mohamed Abd El-Mottleb2, Heba Ismail Khatab3*
1Department of Pharmacology, Faculty of Veterinary Medicine, Zagazig University, 44511, Zagazig, Egypt; 2Department of Pharmacology, Faculty of Medicine, Zagazig University, Egypt; 3Al-Ahrar Teaching Hospital, Ministry of Health, Zagazig, Egypt.
Abstract | This work was carried out to study the potential protective effects of vitamin E and Panax ginseng in male rats against the adverse effects of levofloxacin. Twenty five male rats were haphazardly allocated into five equal groups, group (1) was retained as non-treated control, group (2) given olive oil (0.2 ml/kg b. wt.), group (3) received levofloxacin (10 mg/kg b. wt.), group (4) administered vitamin E (100 ml/kg b. wt.) and levofloxacin (10 mg/kg b. wt.), and group (5) received Panax ginseng (200 ml/kg b. wt.) and levofloxacin (10 mg/kg b. wt.). All doses were given orally once a day for 14 consecutive days. Blood samples were obtained for different hematological and biochemical studies, while the histopathological investigations were conducted using liver and kidney samples. Results showed that levofloxacin administration significantly (P < 0.05) declined erythrocytes count, hemoglobin content, packed cell volume (PCV), platelets (PLT) count and increased leukocytes (WBCs) count compared to group 1 (control). It also significantly elevated liver enzymes (ALT, AST and ALP), total cholesterol (TC), triglycerides (TG), low density lipoprotein (LDL), very low density lipoprotein (VLDL), urea, malondialdehyde (MDA) and tumor necrosis factor-alpha (TNF-α), with reduction in serum high density lipoprotein (HDL), total proteins, albumin, catalase (CAT) and superoxide dismutase (SOD) levels compared to control group. Rats administrated vitamin E or Panax ginseng (groups 4& 5) have significant improvements in all hematological, biochemical and antioxidant parameters that were also confirmed by histopathological findings. The existing investigation concluded that levofloxacin administration induced hematological and biochemical alterations which were ameliorated by using vitamin E and Panax ginseng.
Keywords | Levofloxacin, vitamin E, Panax ginseng, liver functions
Received | May 01, 2021; Accepted | May 21, 2021; Published | November 15, 2021
*Correspondence | Heba Ismail Khatab, Al-Ahrar Teaching Hospital, Ministry of Health, Zagazig, Egypt; Email: hebakhatab87@yahoo.com
Citation | Ibrahim HAE, Mahmoud NM, Abd El-Mottleb DM, Khatab HI (2021). Ameliorative effect of vitamin e and panax ginseng against some adverse effects of levofloxacin in male rats. J. Anim. Health Prod. 9(4): 512-523.
DOI | http://dx.doi.org/10.17582/journal.jahp/2021/9.4.512.523
ISSN | 2308-2801
Copyright © 2021 Khatab et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Levofloxacin is a third-generation, strictly synthetic flouroquinolone antibiotic. It has bactericidal activity against a wide variety of bacteria that are gram positive (G+ve) and gram negative (G-ve) (Al-Soufi and Al-Rekabi, 2019). The principal target of quinolones is DNA gyrase, an essential bacterial enzyme (Abdel-Alim et al., 2017). Several studies have recorded that the most common serious effects associated with fluoroquinolones are headache, diarrhea, oxidative stress, hemolytic anemia, liver and kidney cellular damages (Owens and Ambrose, 2005; Talla and Veerareddy, 2011; Afolabi and Oyewo, 2014). Levofloxacin elevates malondialdehyde (MDA) and nitric oxide (NO) levels in addition to decrease in the level of glutathione in brain areas (Gupta et al., 2009). Also, it raises the activity of liver enzymes due to hepatic diseases (Eyrisofla et al., 2015).
There is a great need for finding protective substances to decrease levofloxacin side effects. Vitamin E is a powerful exogenous antioxidant can protect cell membranes against free radical damage (Bharrhan et al., 2010). Also, it is well known as free radical scavenger and protects cell membranes from oxidation by reacting with lipid radicals produced in the lipid peroxidation chain reaction (Wang et al., 2010). Examples of endogenous antioxidants with a high catalytic effect are SOD and CAT, which are greatly concentrated in all tissues to protect cells from oxidative stress (Morikawa et al., 2000). Generally, vitamin E is commonly used as a medication for the treatment of some health conditions such as muscular dystrophies and certain nervous disorders, male infertility (Keskes-Ammar et al., 2003; Muller, 2010), cardiovascular disease (Lee et al., 2005), enhanced action of insulin in diabetic patients (Pavithra et al., 2018), platelet adhesion inhibition (Gonzalez-Correa et al., 2005) and ageing (Gonzalez-Correa et al., 2005).
Several investigations have been performed on the beneficial effects of medicinal plants (El-Karim et al., 2017; Hashem et al., 2020c). Panax ginseng is also a known plant with a strong antioxidant properties and a wide range of actions such as anti-aging, immune enhancing, anti-stress and anti-cancerous (Cheng et al., 2006). This has re-inforced it’s as a potential antioxidant supplement (Kim et al., 2011). Panax ginseng alleviates oxidative stress by scavenging of free radicals and induction of SOD and CAT activity, thus preventing lipid peroxidation (Chang et al., 1999). Also, it raises SOD, CAT, glutathione peroxidase, and GSH activities in the aged rat liver (Ramesh et al., 2012) and hepatotoxin-induced injury to rat’s liver (Abdul-Hamid et al., 2020).
The existing work was carried out to study the possible ameliorative effects of vitamin E and Panax ginseng on the adverse effects of levofloxacin.
Materials and methods
Drugs
Levofloxacin (TAVANIC®) tablets were obtained from Sanofi Aventis Company, Egypt. Each tablet contains 500 mg levofloxacin, dissolved in DW (distilled water) and taken by oral route in a dosage of 10 mg/kg b.wt. (Olayinka et al., 2015). Vitamin E (VITAMIN E®) capsules were obtained from Pharco Pharmaceuticals Company, Egypt. Each capsule contains 1000 mg vitamin E, dissolved in a vehicle, olive oil and orally administered (100 mg/kg b.wt.) as standard antioxidant according to Ambali et al. (2011). Panax ginseng (GINSANA®) capsules were obtained from EIPICO Pharmaceuticals Company, Egypt, dissolved in DW and administered orally at a dosage of 200 mg/kg b.wt. according to Esawe (2017). All medications were administered orally once a day for 14 consecutive days.
Ethical statements
The care and revival of the rats used was consistent with the strategies in Research Committee at Zagazig University, Egypt.
Experimental animals
Twenty five adult apparently healthy male albino rats (weighing 200±10g) were attained from the Laboratory House of Experimental Animal, Faculty of Veterinary Medicine, Zagazig University, Egypt. Rats were accustomed prior to the experiment for two weeks. In well ventilated plastic cages containing hard wood bedding and good aeration, the animals were housed at a temperature (25 ± 5°C) normal dark/light cycle of 12h, humidity (55±%). During the investigation, the rats were supplied with a known basal diet composition and water ad-libitum.
Experimental design
Rats were divided into five equal groups of 5 rats each; group 1 received 0.2 ml/kg b.wt. of distilled water (normal control group), group 2 received 0.2 ml/kg b.wt. of olive oil (vehicle control), group 3 received 10 mg/kg b.wt. of levofloxacin, group 4 received 100 ml/kg b.wt. of vitamin E and 10 mg/kg b.wt. of levofloxacin while group 5 received Panax ginseng (200 ml/kg b.wt.) and levofloxacin (10 mg/kg b.wt.). All doses were taken once a day via oral gavage for 14 successive days to all the groups. Vitamin E or Panax ginseng was provided 2 hours prior to the administration of levofloxacin.
Sampling
At the experiment termination, 5ml sample blood obtained from each rat using heparinized microhematocrite tubes through the retro-orbital venous plexus was separated into two portions (Hashem et al., 2018). The 1st part was put in tubes containing EDTA as anticoagulant for hematological studies using Hemascreen 18 Automatic Cell Counter (Hospitex Diagnostics, Sesto Fiorentino, Italy) and 2nd part into plain tubes without anticoagulant to obtain clear serum for biochemical analysis. Serum was analyzed for estimation of liver enzymes (AST, ALT and ALP), total proteins, albumin, globulin and albumin/globulin (A/G) ratio, lipids profile (cholesterol, TG, LDL, VLDL and HDL) and kidney function markers (urea and creatinine) utilizing a entirely automated analyzer for chemistry (New Delhi, India). In addition, serum antioxidants (SOD and CAT) and MDA as oxidative stress were determined on UV-Vis Spectrophotometer (Apple 303S, Japan), while TNF-α, proinflammatory marker, was measured by ELISA using ELISA kit Catalog number: MBS702888. All biochemical parameters were evaluated by means of viable test kits obtained from Biodiagnostic Co., Cairo, Egypt as instructed by the manufacturer.
Tissue samples were taken from the liver and kidneys of euthanized rats by manual cervical dislocation and fixed in neutral formalin of 10 %. The samples preserved with formalin are dehydrated and embedded in paraffin. Five- micron thick paraffin slices were set and tainted with haematoxyline and eosin (H& E) and inspected microscopically (Suvarna et al., 2018).
Statistical analysis
After obtaining the data, it was statistically analyzed by ANOVA using SPSS computer program (Tamhane and Dunlop, 2000) for windows version16.
Results
Hematological results
Erythrogram: Existing study showed significantly (P<0.05) reduction in erythrocyte counts, Hb concentration, PCV and PLT count in rats administered levofloxacin in related to control group. However, erythrocyte indices (MCV and MCHC) revealed non-significant alterations. Oral administration of vitamin E or Panax ginseng before administration of levofloxacin by 2hours (groups 4&5) produced a significant rise and improvement for the erythrocyte parameters and PLT count if matched with levofloxacin group (Table 1).
Leukogram: The present study revealed that levofloxacin elicited a significant (P < 0.05) intensification in WBCs, lymphocytes, neutrophils and monocytes counts in relation to group 1. Administration of vitamin E or Panax ginseng before levofloxacin by 2hours (groups 4&5) in male rats displayed a significant decreases in the aforementioned parameters in consideration with levofloxacin group (Table 2).
Serum biochemistry
Liver enzymes: The present investigation indicated that administration of levofloxacin in male rats displayed an elevation significantly (P < 0.05) in ALT, AST and ALP activities when compared with control group while administration of vitamin E or Panax ginseng with levofloxacin (groups 4&5) displayed a noticeable decrease in ALT, AST and ALP activities in comparison to levofloxacin group (Table 3).
Serum proteins: Oral administration of levofloxacin in male rats produced a significant (P<0.05) hypoproteinemia with a diminution in albumin, globulins and A/G ratio. These values were significantly improved after supplementation of vitamin E or Panax ginseng before levofloxacin by 2 hours (groups 4&5) compared to the levofloxacin group (Table 4).
Lipid profile: Levofloxacin given orally in male rats elicited a significant (P < 0.05) rise in all estimated lipid fractions except HDL levels which decreased when compared to control (group1). Vitamin E or Panax ginseng administration before levofloxacin by 2hours (groups 4&5) led to discernible falling in lipogram with a significant high HDL levels compared with levofloxacin group (Table 5).
Kidney function markers: Oral administration of levofloxacin in male rats produced a significant (P < 0.05) upsurge in urea and creatinine levels when compared to control (group1). However, supplementation of vitamin E or Panax ginseng before levofloxacin by 2hours (groups 4&5) in male rats displayed a statistical decline in urea and creatinine levels when compared to the levofloxacin group (Table 6).
Oxidant/Antioxidant status: Rats taken levofloxacin (group3) exhibited at probability <0.05 intensification in MDA concentration, with lessening in the activities of CAT and SOD related to control. Vitamin E- or Panax ginseng- levofloxacin supplementation (groups 4&5) displayed declines in MDA concentration with elevated CAT and SOD activities associated with levofloxacin group (Table 7).
Tumor necrosis factor-α: As illustrated in Table (8) levofloxacin exposure produced a significant rise in serum TNF-α in assessment with group (1). Oral administration of vitamin E or Panax ginseng before administration of levofloxacin by 2hours (groups 4&5) elicited a significant drop in TNF-α matching with 3rd group. Non-significant variation in TNF-α were recorded post administration of Panax ginseng and levofloxacin (group5).
Histopathological results
Sections from liver of control (group1) exhibited typical liver parenchyma, such as normal hepatocytes and sinusoids morphology, as well as usual hepatic lobules and ducts (Figure 1 A&B). Kidney sections (group1) revealed normal Bowman’s capsules, glomeruli and tubules structure (Figure 1 B&C).
Liver sections from rats given olive oil (group 2) showed ordinary hepatic structure with preserved lobular pattern, vascular tree, kupffur cells and stromal component (Figure 2 A&B). Kidney sections (group2) revealed normal
Table 1: Effect of oral administration of vitamin E (100 mg/kg b.wt.) or Panax ginseng (200 mg/kg b.wt) on erythrogram of rats exposed to levofloxacin (10 mg/kg b.wt.) for 14 successive days. (Mean ± SE) (n = 5)
| Groups | Erythrogram | |||||
|
RBCs (x106 /µl) |
Hb (g/dl) |
PCV (%) |
MCV (Fl) |
MCHC (pg) |
PLT (x103 /µl) |
|
| Control |
8.07a ± 0.12 |
15.10a 0.31 |
56.66a 1.20 |
70.23a ± 0.69 |
28.41a ± 0.35 |
336.00a ± 8.19 |
| Olive oil |
8.00a 0.03 |
15.56a 0.34 |
54.66a 1.20 |
68.32a ± 1.2 |
28.47a ± 0.39 |
331.00a ± 15.09 |
| Levofloxacin |
4.91d ± 0.05 |
9.67d ± 0.36 |
34.75d ± 1.11 |
70.68a ± 1.56 |
27.98a ± 0.57 |
258.75c ± 10.63 |
| Vitamin E+ Levofloxacin |
6.89b 0.04 |
13.62b 0.17 |
46.50b 1.65 |
67.49a ± 1.15 |
24.31a ± 0.59 |
282.50b ±10.91 |
|
Panax ginseng + Levofloxacin |
5.71c 0.04 |
11.40c 0.18 |
40.75c 0.85 |
71.35a ± 1.34 |
28.00a ± 0.63 |
293.25b ± 5.85 |
Means within the same column carrying different superscript letters are significant at P < 0.05
RBCs: Red blood cells count, Hb: Hemoglobin, PCV: packed cell volume, MCV: Mean corpuscular volume, MCH: Mean corpuscular hemoglobin, MCHC: Mean corpuscular hemoglobin concentration, PLT: platelet count
Table 2: Effect of oral administration of vitamin E (100 mg/kg b.wt.) or Panax ginseng (200 mg/kg b.wt.) on leukogram of rats exposed to levofloxacin (10 mg/kg b.wt.) for 14 successive days. (Mean ± SE) (n = 5)
| Groups | Leukogram | ||||
|
WBCs (x103/µl) |
Lymphocytes (x103 /µl) |
Neutrophils ( x103 /µl) |
Eosinophils ( x103 /µl) |
Monocytes ( x103 /µl) |
|
| Control |
11.08d ± 0.24 |
8.59c ± 0.24 |
1.34d ± 0.05 |
0.44a ± 0.018 |
0.72d ± 0.02 |
| Olive oil |
11.01d ± 0.03 |
8.51c ± 0.49 |
1.35d ± 0.03 |
0.44a ± 0.03 |
0.71d ± 0.03 |
| Levofloxacin |
18.49a ± 0.59 |
11.89a ± 0.48 |
4.44a ± 0.20 |
0.42a ± 0.07 |
1.75a ± 0.09 |
| Vitamin E+Levofloxacin |
13.33c ± 0.45 |
8.47b ± 0.39 |
2.51c ± 0.22 |
0.44a ± 0.03 |
0.92c ± 0.01 |
|
Panax ginseng +Levofloxacin |
15.16b ± 0.22 |
9.37b ± 0.22 |
3.29b ± 0.28 |
0.42a 0.05 |
1.09b ± 0.02 |
Means within the same column carrying different superscript letters are significant at P < 0.05
WBCs: White blood cells count
Table 3: Effect of oral administration of vitamin E (100 mg/kg b.wt.) or Panax ginseng (200 mg/kg b.wt.) on serum liver enzymes activity of rats exposed to levofloxacin (10 mg/kg b.wt.) for 14 successive days. (Mean ± SE) (n = 5)
| Groups |
Liver enzymes |
||
|
ALT (U/l) |
AST (U/l) |
ALP (U/l) |
|
| Control |
13.67d ± 1.20 |
14.67d ± 0.88 |
72.10d ± 0.05 |
| Olive oil |
13.00d ± 1.54 |
14.00d ± 1.73 |
71.87d ± 1.47 |
| Levofloxacin |
69.25a ± 2.14 |
57.50a ± 1.55 |
147.22a ± 2.25 |
| Vitamin E + Levofloxacin |
40.00c ± 1.29 |
34.50c ± 2.63 |
99.95c 1.8268 |
|
Panax ginseng + Levofloxacin |
57.00b ± 1.58 |
43.75b ± 1.38 |
127.65b ± 2.62 |
Means within the same column carrying different superscript letters are significant at P < 0.05.
ALT: Alanine aminotransferase, AST: Aspartate aminotransferase, ALP: Alkaline phosphatase.
Table 4: Effect of oral administration of vitamin E (100 mg/kg b.wt.) or Panax ginseng (200 mg/kg b.wt.) on serum proteins profile of rats exposed to levofloxacin (10 mg/kg b.wt.) for 14 successive days. (Mean ± SE) (n = 5)
| Groups | Serum proteins | |||
|
Total Proteins (g/dl) |
Albumin (g/dl) |
Globulins (g/dl) |
A/G ratio
|
|
| Control |
7.30a ± 0.11 |
5.15a ± 0.06 |
2.15a ± 0.09 |
2.39ab ± 0.12 |
| Olive oil |
7.18a ± 0.09 |
5.19a ± 0.16 |
1.99a ± 0.25 |
2.72a 0.47 |
| Levofloxacin |
5.19d ± 0.09 |
2.97d ± 0.11 |
2.23a 0.11 |
1.34c ± 0.11 |
| Vitamin E+ Levofloxacin |
6.36b ± 0.07 |
4.19b ± 0.05 |
2.16a 0.09 |
1.96bc ± 0.11 |
|
Panax ginseng + Levofloxacin |
6.01c ±0.05 |
3.52c ± 0.16 |
2.15a ±0.19 |
1.787bc ± 0.22 |
Means within the same column carrying different superscript letters are significant at P < 0.05
A/G ratio: Albumin/ globulin ratio
Table 5: Effect of oral administration of vitamin E (100 mg/kg b.wt.) or Panax ginseng (200 mg/kg b.wt.) on serum lipids profile of rats exposed to levofloxacin (10 mg/kg b.wt.) for 14 successive days. (Mean ± SE) (n = 5)
| Groups | Lipid profile | ||||
|
Total cholesterol (mg/dl) |
Triglycerides (mg/dl) |
HDL (mg/dl) |
LDL (mg/dl) |
VLDL (mg/dl) |
|
| Control |
92.00d ± 1.53 |
146.33d ± 4.05 |
68.33a ± 4.91 |
54.21d ± 4.81 |
29.27d ± 0.82 |
| Olive oil |
93.00d ± 2.08 |
147.00d ± 2.65 |
66.67a ± 3.76 |
56.91d ± 5.37 |
24.40d ± 0.53 |
| Levofloxacin |
193.75a ± 1.49 |
259.25a ± 3.22 |
23.500d ± 1.443 |
239.90a ± 1.27 |
51.85a ± 0.64 |
| Vitamin E+ Levofloxacin |
139.25c ± 2.29 |
199.50c ± 4.41 |
45.500b 1.5545 |
142.65c ± 2.75 |
39.90c ± 0.88 |
|
Panax ginseng + Levofloxacin |
164.75b ± 2.14 |
236.00b ± 2.27 |
33.250c ± 1.7017 |
194.43b ± 3.93 |
47.20b ± 0.45 |
Means within the same column carrying different superscript letters are significant at P < 0.05
HDL: High density lipoprotein, LDL: Low density lipoprotein, VLDL: Very low density lipoprotein.
Table 6: Effect of oral administration of vitamin E (100 mg/kg b.wt.) or Panax ginseng (200 mg/kg b.wt.) on serum kidney function parameters of rats exposed to levofloxacin (10 mg/kg b.wt.) for 14 successive days. (Mean ± SE) (n = 5)
| Groups | Kidney functions | |
|
Urea (mg/dl) |
Creatinine (mg/dl) |
|
| Control |
27.47d± 0.99 |
0.79d± 0.03 |
| Olive oil |
27.33d± 0.49 |
0.79d± 0.02 |
| Levofloxacin |
72.75a± 1.15 |
2.03a± 0.05 |
| Vitamin E + Levofloxacin |
39.85c± 1.27 |
1.01c± 0.04 |
|
Panax ginseng + Levofloxacin |
51.03b± 0.52 |
1.23b± 0.03 |
Means within the same column carrying different superscript letters are significant at P < 0.05
TNF-α: Tumor necrosis factor-alpha.
Table 7: Effect of oral administration of vitamin E (100 mg/kg b.wt.) or Panax ginseng (200 mg/kg b.wt.) on oxidant/antioxidant status of rats exposed to levofloxacin (10 mg/kg b.wt.) for 14 successive days. (Mean ± SE) (n = 5)
| Groups | Oxidant/antioxidant status | ||
|
MDA (mmol/ml) |
CAT (u/l) |
SOD (u/ml) |
|
| Control |
11.47d ± 0.49 |
501.32a ± 9.43 |
3.84a ± 0.08 |
| Olive oil |
12.43d ± 0.33 |
506.25a ± 7.29 |
3.82a ± 0.08 |
| Levofloxacin |
73.75a ± 1.59 |
311.09c ± 4.08 |
1.01d ± 0.04 |
| Vitamin E + Levofloxacin |
35.93c ± 1.83 |
410.75b ± 6.79 |
1.48b ± 0.03 |
|
Panax ginseng + Levofloxacin |
56.24b ± 1.61 |
362.62c ± 3.95 |
1.42c ± 0.05 |
Means within the same column carrying different superscript letters are significant at P < 0.05.
MDA: Malondialdehyde, CAT: Catalase, SOD: Superoxide dismutase.
nephron units with preserved glomerular and tubular structures (Figure 2 B&C).
Exposure to levofloxacin (group3) produced slight to reasonable congestion of portal veins, central veins and hepatic sinusoids. Moderate biliary proliferation and round aggregations of cells have been seen. Some hepatocytes showed degenerative changes mainly vacuolations and few
Table 8: Effect of oral administration of vitamin E (100 mg/kg b.wt.) or Panax ginseng (200 mg/kg b.wt.) on serum TNF-α of rats exposed to levofloxacin (10 mg/kg b.wt.) for 14 successive days. (Mean ± S.E) (n = 5)
| Groups |
TNF-α (pg/ml ) |
| Control |
19.23d± 0.90 |
| Olive oil |
20.03d± 0.55 |
| Levofloxacin |
90.80a± 1.45 |
| Vitamin E + Levofloxacin |
51.30c± 1.01 |
|
Panax ginseng + Levofloxacin |
71.80b± 0.63 |
Means within the same column carrying different superscript letters are significant at P < 0.05. TNF-α: Tumor necrosis factor-alpha.
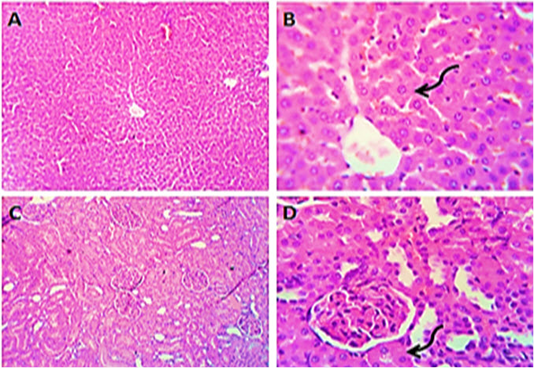
Figure 1: Photomicrographs of liver (A&B) and kidney (C&D) sections from control group (group1) showing normal histo-morphologic structures with mild degenerative changes in some hepatocytes and some of the renal tubular epithelium (curved arrows). H&E. Magnification x100 (A, C) & x400 (B, D).
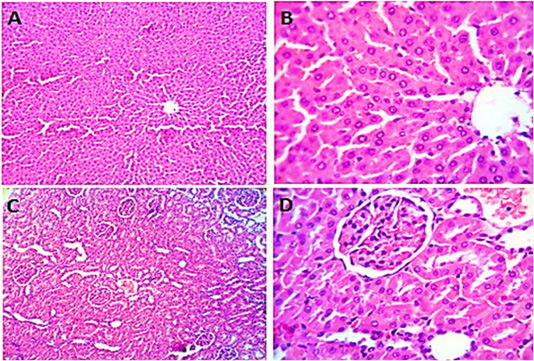
Figure 2: Photomicrographs of liver sections (group2) from male rats given olive oil showing normal hepatic parenchyma with preserved lobular pattern, portal triads structures, vascular tree, kupffur cells and stromal component (A, B). Kidney sections (group2) of male rats received olive oil showing normal nephron units with preserved glomerular and tubular structures, normal limits with normal histomorphology of the blood vessels and stroma (C, D). H&E. Magnification x400.
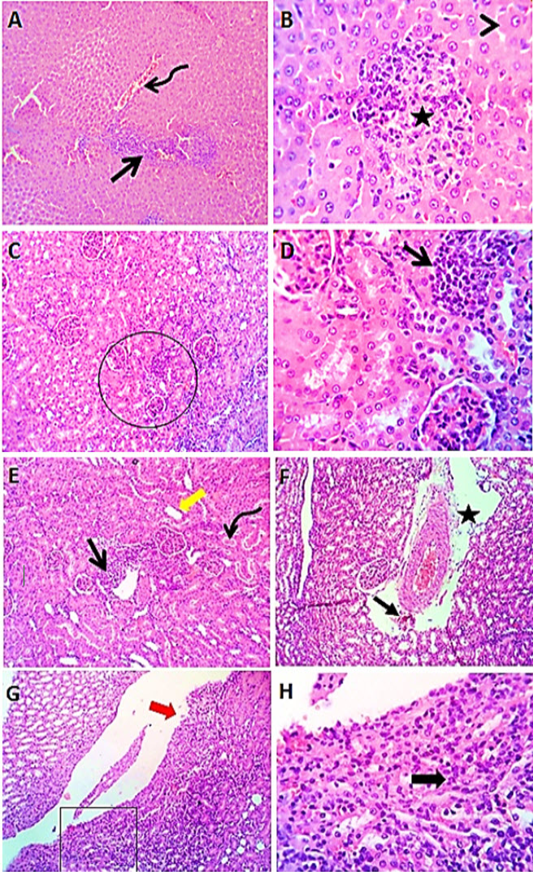
Figure 3: Photomicrographs of liver sections (group3) from male rats received levofloxacin showing moderate congestion of portal blood vessels (curved arrow) with round cells aggregations (open arrow), focal interstitial round cells aggregation (star) with degenerative changes (arrow head) in some hepatocytes (A & B). Kidney sections (group3) of male rats exposed to levofloxacin showing lymphocytic interstitial aggregations (open arrows), perivascular edema (star) sometimes with minute hemorrhages (closed arrow), dilated renal tubules (thick yellow arrow) in both cortex and medulla with intratubular hyaline cast (curved arrow). The renal pelvis and the renal papillae showing lymphocytic pyleitis (square) with superficial ulceration of the lining epithelium (red thick arrow) and moderate subepithelial lymphocytic aggregation (black thick arrow) (C- H). H&E. Magnification x100 (A, C, E, G) & x400 (B, D, F, H).
apoptotic cells (Figure 3 A&B). Kidney sections (group3) revealed renal lesions such as interstitial, perivascular and periglomerular lymphocytic aggregations, with dilated renal tubules in both cortex and medulla with hyaline casts (Figure 3 C-H).
Liver sections from rats administered vitamin E before the administration of levofloxacin by 2 hours (group4) revealed an enhancing vitamin E result which appeared as discount in number of lymphocytic aggregation in the portal area. Most of hepatocytes appeared normal (Figure 4 A- E). Kidney sections (group4) showed apparently normal renal tubules with few renal tubular epithelial cells showing vacuolar degeneration (Figure 4 F- H).
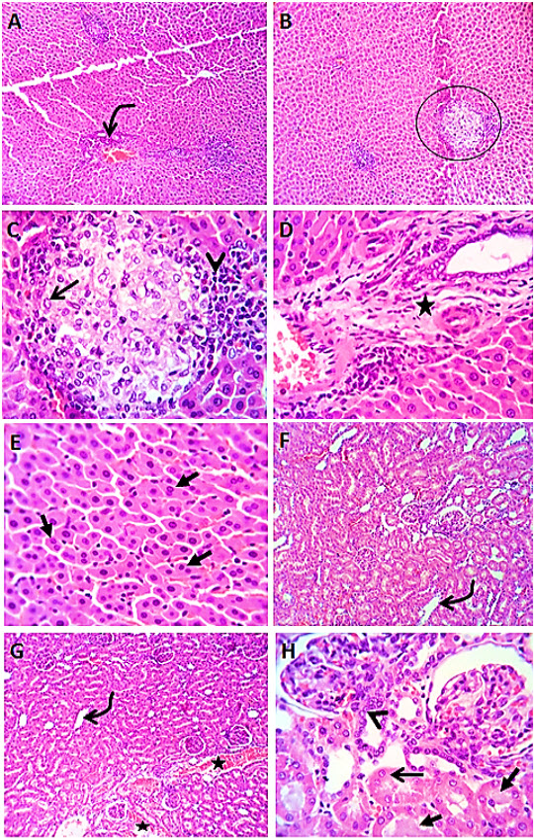
Figure 4 Photomicrographs of liver sections (group4) from male rats given vitamin E before the administration of levofloxacin by 2hours showing lymphocytic aggregation in the portal areas (curved arrow) with healing reaction in both the portal area (star) and the interstitial tissue (circle), with replacement of the lymphocytes (arrow head) by macrophages (open arrow) or fibroblasts. Most of the hepatic parenchyma showing active hepatocytes with increased number of binucleated cells (closed arrows) (A-E). Kidney sections (group4) showing mild congestion (stars) of renal blood vessels, dilatation of some cortical and medullary tubules (curved arrows) beside regenerative attempts in some tubules (arrow head). A few tubules showing cloudy swelling (open arrow) and /or hyaline casts (closed arrows) (F- H). H&E. Magnification x100 (A, B) & x400 (C, D, E, F, G, H).
Liver sections from rats administered Panax ginseng before the administration of levofloxacin by 2 hours (group5) revealed apparently normal hepatocytes with some binucleated hepatocytes, beside mild lymphocytic inflammatory reaction (Figure 5 A&B). Kidney sections (group5) showed mildly congested renal blood vessels and normal medulla. Some renal tubules in both cortex and medulla were dilated (Figure 5 C&D).
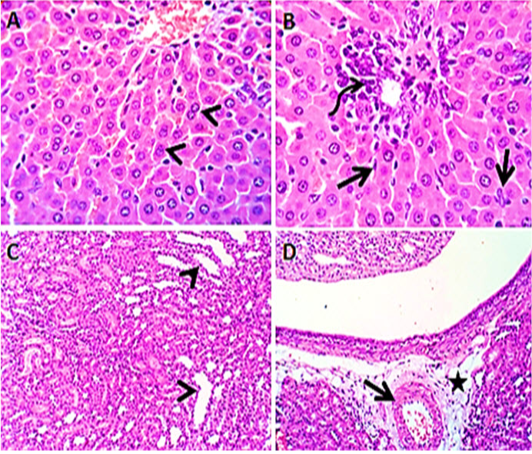
Figure 5: Photomicroraph of liver sections (group5) from male rats given Panax ginseng before the administration of levofloxacin by 2hours showing some binucleated hepatocytes (arrow heads) with activated kupffer cells (open arrow), beside mild lymphocytic reaction (curved arrow) in the portal area (A &B). Kidney sections (group5) showing mildly congested renal blood vessels (open arrow) with perivascular edema (star) and some renal tubules in both cortex and medulla are dilated (arrow heads) (C & D). H&E. Magnification x100 (C, D) & x400 (A, B).
Discussion
Levofloxacin is a flouroquinolone antibiotic of 3rd generation and an optical S-(-) isomer of the racemic drug substance ofloxacin. It has a wide range of action against G+ve and G-ve bacteria, as well as other pathogens, for example Mycoplasma, Legionella, Chlamydia, and Mycobacteria species (Riahifard et al., 2017).
The existing work revealed that levofloxacin exposure for 14 successive days in male rats caused leukocytosis as seen in the significant increase in WBCs, lymphocytes, neutrophils and monocytes counts with associated anemia due to diminution in values of RBCs, Hb and percentage levels of PCV, with decreased PLT count (thrombocytopenia) matching with control (group1). This alteration in the erythrocytic parameters may be due to inhibitory influence of levofloxacin on the erythropoiesis in bone marrow (Hashem et al., 2020a). The observed thrombocytopenia may be attributed to either reduced bone-marrow depltion platelet production or increased drug-mediated destruction (Shih et al., 2018). In particular, levofloxacin may have minor blood clotting defects, which have reduced the platelets number in dogs during the treatment course (Psarros et al., 2014). The results of leukogram may be due to some drugs as levofloxacin specifically stimulate truthful autoantibodies production as those seen in autoimmune hemolytic anemia with high formation of lymphocytes (Psarros et al., 2014). The increases in neutrophil and monocyte counts are as a defense mechanism against inflammation (Weiss and Wardrop, 2011). Similar observations were reported following oral administration of levofloxacin in rabbits (Khan et al., 2017) or in male rats (Tohamy, 2017).
Oral administration of vitamin E or Panax ginseng before levofloxacin by 2 hours (groups 4&5) for 14 successive days ameliorated these adverse effects caused by levofloxacin as observed in the noticeable elevation in erythrocyte count, Hb content, and PCV % and PLT count compared to levofloxacin- exposed rats (group 1). The enhanced erythrogram and leukogram post-administration of Panax ginseng in rats treated with levofloxacin may be endorsed to the Panax ginseng anti-oxidants activity (Kim et al., 2011). In addition, vitamin E has contributed to increase in the number of erythroid precursor units forming colonies, preventing polyunsaturated fatty acid oxidation in the erythrocyte membrane, inhibiting premature erythrocyte lysis and improving erythropoiesis. Vitamin E can thus enhance post-supplemental Hb content and percentage levels of PCV (Jilani and Iqbal, 2011). The obtained results were supported by earlier reports (Mahran, 2013; Tohamy, 2017), in which vitamin E supplementation with levofloxacin in rats resulted in a significant increase in total erythrocyte counts, Hb content and PCV % and insignificant drop in TLC is counted on the 1st and 10th days after administration of the drug and restored to almost normal levels on the 20th day following administration.
A significant increase was detected in the activities of ALT, AST and ALP enzymes after administration of levofloxacin in male rats for 14 successive days. The elevation in the enzymatic activities may be related to hepatic malfunction (Ara et al., 2020) or due to increased breakdown of hepatocytes (Hashem et al., 2020a,b). The pathological changes, round cells aggregation with degenerative changes in hepatocytes, in the liver have long-established this results. Our findings was clearly supported by Olayinka et al. (2015) and Tohamy (2017) who mentioned that oral administration of levofloxacin (7.5 mg/kg b.wt.) in rats displayed rise in enzymes of the liver on the 1st and 10th days and return nearly to normal levels on 20th day post administration in parallel to control (group1). Moreover, Farid and Hegazy (2019) stated that levofloxacin- administered rats for 2 weeks (40 mg / kg b. wt.) daily revealed hepatic dysfunction with increased in AST &ALT activities. The current study indicated that co-administration of vitamin E or Panax ginseng with levofloxacin in male rats declined the previous results for enzymes in consistent with the levofloxacin group. Our results come to an agreement with findings of Liu et al. (2018) who reported that administration of Panax ginseng in alcohol treated rats improved liver functions (ameliorated the elevated AST, ALT and ALP activities), and Hashem et al. (2019) who exposed that dosing antioxidants like vit. E to diet with cadmium significantly restored the activities of serum AST, ALT to normal values. Similar results were recorded by George and Adegoke (2011) who found that vitamin E displayed a reduction in liver transaminases, GGT &ALP activity in albino rats, and Tohamy (2017) who stated the same findings after administration of vitamin E in male rats. The obtained results in vitamin E or Panax ginseng- supplemented rats were supported by histopathological findings.
In this experiment, oral administration of levofloxacin in male albino rats produced a hypoproteinemia, hypoalbuminemia, and reduction in the ratio of A/G, with insignificant globulins changes compared to the normal control. The hypoproteinemia and hypoalbuminemia may be attributed to liver and kidney damage caused by high dose of levofloxacin. Moreover, hypoalbuminemia may be a result of decreased production of albumin from damaged liver or increased loss of albumin via from damaged kidneys (Braun and Lefebvre, 2008). The increased A/G ratio in this study is due to hypoalbuminaemia caused by levofloxacin - exposure with normal total globulins level. Liver disease is the cause of reduction in plasma albumin, α- and ß-globulins with a rise in the level of γ–globulins (David Eckersall, 2008), this explains the unchanged total globulins levels as end result in rats with hepatic damage. The results in proteinogram were significantly improved after supplementation of vitamin E or Panax ginseng matched to the levofloxacin group. These results confirm the hepatoprotective role of vitamin E or Panax ginseng against levofloxacin- induced liver damage. Similar findings were reported by Abdelfattah-Hassan et al. (2019).
Levofloxacin administration causes dyslipidemia evidenced by numerical augmentation in all measured lipid profile in this work, with drop in HDL comparatively with control group. This may be attributed to liver damage and nephrotoxic effect induced by levofloxacin (Oda et al., 2014). Moreover, the alterations in lipids profile indicate lipoproteins and lipids metabolism disorders and might contribute to the development of phospholipidosis and cholesterogenesis in tissues which might represent additional adverse effects of levofloxacin (Owoade et al., 2018). Our results are consistent with Olayinka et al. (2015), described a numerical rise in serum lipogram with levofloxacin administration.
The current study revealed co-administration of vitamin E or Panax ginseng with levofloxacin resulted in alleviation in lipid profile compared to administration of levofloxacin alone. This can be due to a decline in the damaging effects of ROS and nitrogen molecules produced by levofloxacin administration (Talla and Veerareddy, 2011).
Serum urea and creatinine levels are widely used as markers for renal function screening as they are the most sensitive parameters in diagnosis of renal disease (Ferguson and Waikar, 2012). Levofloxacin administartion produced a remarkable upsurge in urea and creatinine levels. The increase in estimated renal bio-markers might be attributed to a reduction in the filtration rate of glomeruli or subordinate to the increase of ROS induced-renal damage and decreased renal excretory function (Noori and Mahboob, 2010). Improved protein catabolism along with accelerated gluconeogenesis amino acid deamination is possibly an appropriate propose for the understanding of elevated urea levels (Hashem and El-Sharkawy, 2009). The obtained outcomes correspond to those obtained by Mouton and Holder (2006), Afolabi and Oyewo (2014) who stated that levofloxacin produces ROS that can cause oxidative stress, with liver and kidney cellular damage. Our findings were supported by the pathological examination kidneys sections of rats exposed to levofloxacin only, which showed interstitial lymphocytic aggregations, perivascular edema with mild hemorrhages as well as, dilated renal tubules with intratubular hyaline cast. Similar findings were obtained by Oda et al. (2014) who reported that levofloxacin treated rabbits showed diffuse lymphocytic interstitial nephritis, tubular necrosis and some hyalinized and necrotic tubules. Moreover, Ara et al. (2020) showed that the administration of levofloxacin in mice induced renal damage, as evidenced by glomerulonephritis, degenerations of epithelium and tubules degeneration with tubule dilation. However, co-administration of vitamin E or Panax ginseng with levofloxacin displayed diminution in kidney function parameters in correlated to the group treated with levofloxacin only. The same result was also obtained by George and Adegoke (2011) who reported that rats fed diet containing vitamin E showed reduction in urea and creatinine levels. The reduction in kidney function tests after supplementation of vitamin E with levofloxacin was due to its antioxidant effect, the scavenging activity of free radicals, and the defense of protein thiols from the deleterious effect of levofloxacin in the kidney (Hashem et al., 2019). While Panax ginseng containing phenolic acids, flavonoids and saponins are the cause of improvement in the function of kidney through dissimilar antioxidant characteristics corresponding to scavenging action of free radical or oxidized product formation inhibition (Kalkan et al., 2012).
There was also a significant rise in MDA concentrations with a significant decline in CAT & SOD enzymes in rats administered levofloxacin alone in this study, indicative of induction of oxidative stress by the drug. A biomarker of tissue damage has been identified as an elevation in the MDA level (Gutteridge, 1995). Antioxidant enzymes are inactivated by MDA as one of lipid peroxidation products resulting in augmented ROS addition and worsening macromolecular damage (Noeman et al., 2011). The depletion of antioxidant enzymes such as SOD and CAT was attributed to levofloxacin-induced oxidative cell damage as a consequence of the production of reactive free radicals that overpower the antioxidant protection (Talla and Veerareddy, 2011). Our findings were consistent with previous results in levofloxacin- exposed rabbits (Khan et al., 2017) or immature albino rats (Abd Elfadil et al., 2019). Moreover, the observed data in this study corroborates with the work of Farid and Hegazy (2019) who reported that levofloxacin administration in rats displayed oxidative- stress represented by lessening in antioxidant enzymes (CAT & SOD) with high MDA concentrations.
Co-administration of vitamin E or Panax ginseng with levofloxacin ameliorated this effect as seen in the decline in MDA concentrations with a concurrent upsurge in CAT and SOD enzyme activities comparing to the levofloxacin group. This suggests the ability of the antioxidant constituents of vitamin E or plants (Panax ginseng) to break lipoperoxidation chain reaction and facilitate the removal of reactive oxygen species generated by levofloxacin over dose (Hashem et al., 2015). The improved antioxidant enzymes (SOD and CAT) activity induced by vitamin E could be validated to the well-established potential or capacity of vitamin E as antioxidant (Helen et al., 2000), as it inhibits toxic effect to the reactive membrane lipids by preventing creation of hydroperoxide. It is reported that vitamin E- selenium administration is recorded to protect cell membranes and organelles-containing lipids from peroxidative destruction (Gupta et al., 2005; Hashem et al., 2019). Administration of Panax ginseng (3g for 12 weeks) was also reported to have produced intensification in SOD level, with a drop in MDA concentration (Seo et al., 2014). Analogous outcomes reported that administration of Panax ginseng in alcohol- treated rats caused statistical elevation in SOD activity and lessening in MDA concentration (Liu et al., 2018).
The present investigation indicated that oral administration of levofloxacin in rats produced a numerical upsurge of TNF-α in the serum in comparison to control group. Tumor necrosis factor is a potent pro- inflammatory cytokine that is high in systemic and local inflammation and is considered essential for the recruitment of neutrophils (Diez-Pina et al., 2009). Similar results were also previously reported (Sharma et al., 2015). Fischer and Marier (2015) found a strong association between the expression of cytokines like TNF-α and oxidative stress in rats. This study may explain the significant reduction in TNF-α level in groups which were treated with vitamin E or Panax ginseng 2 hours before levofloxacin when compared to levofloxacin administration only, as they had powerful antioxidant properties which strongly counteracted any adverse effect produced by oxidative stress such as elevation of TNF-α level in levofloxacin group. Vitamin E significantly decreased serum TNF-α level equated to rats group- treated with vancomycin only in the exploration of Blesa et al. (2003) and attributed that to its direct antioxidant effect, additionally it can protect indirectly via decreasing neutrophil recruitment.
Conclusion
The results obtained from this work showed that levofloxacin administration induced hematological and biochemical alterations which were ameliorated by the vitamin E and Panax ginseng. The Panax ginseng administration exhibited superior antioxidant and antihepato-renal toxic activities compared with vitamin E, that may be attributed to its’ radical scavenging activity.
Conflict of interest
The authors pronounce that they have no conflict of interest.
Availability of data and materials
All author stated that all information produced or analyzed during this work is included.
Funding
This study does not have a funding source.
Acknowledgments
The authors are grateful to all stuff members and workers of Pharmacology Department, Faculty of Veterinary Medicine, Zagazig University, for their valuable assistance during this work.
authors contribution
All authors contributed equally.
References





