Journal of Animal Health and Production
Research Article
Effect of Post-Mating Treatment with Controlled Internal Drug Release (CIDR) on Pregnancy Rate of Repeat Breeder Egyptian Buffaloes
Mohamed Abd El-Fattah Abo-Farw1, Wael Mohamed Nagy1, Abdel-Khalek El-Sayed Abdel-Khalek2*
1Animal Production Research Institute, Agricultural Research Center, Egypt; 2Department of Animal Production, Faculty of Agriculture, Mansoura University, Egypt.
Abstract | To reduce early embryonic loss and improving pregnancy rate (PR) of Egyptian repeat breeder buffaloes, 45 repeat breeder animals were used in this study. Buffalo cows (n= 30) and buffalo heifers (n= 15) were allocated to three groups (10 cows and 5 heifers in each). Animals exhibiting normal heat were naturally mated. On Day 5 post-mating, a Controlled Internal Drug Release (CIDR-B) device was inserted on Day 5-9 (Protocol 1, P1), and Day 5-18 (Protocol 2, P2) post-mating. Animals in the 3rd group (Control) were left without treatment. Blood samples were taken on different days post- mating. Results show overall pregnancy rate (PR) of 46.7, 66.7 and 13.3% in P1, P2 and control (P< 0.05), being higher in buffalo-cows than in buffalo-heifers (46.7 vs. 33.3%, P< 0.05). In controls, PR was 0 in buffalo-heifers and 20% in buffalo-cows. Level of progesterone (P4) was higher (P< 0.05) in P1 and P2 than in control on most post-mating days following CIDR insertion, being higher in P2 than in P1, and higher (P≥ 0.05) in cows than in heifers. Level of P4 at estrus decreased (P < 0.01), while post-mating P4 levels increased for all pregnant in comparing with non-pregnant animals. In conclusion, increasing the progesterone concentrations by CIDR treatment from 5-18 days post-mating could be a useful strategy to prevent or reduce embryo mortality and is recommended for improving pregnancy in repeat breeder buffalo cows and heifers.
Keywords | Buffalo, Repeat breeder, Estrus, Progesterone, Pregnancy
Received | June 01, 2021; Accepted | June 24, 2021; Published | November 15, 2021
*Correspondence |Abdel-Khalek El-Sayed Abdel-Khalek, Department of Animal Production, Faculty of Agriculture, Mansoura University, Egypt; Email: abdelkhalk2004@yahoo.com
Citation | Abo-Farw MA, Nagy WM, Abdel-Khalek AE (2021). Effect of post-mating treatment with controlled internal drug release (cidr) on pregnancy rate of repeat breeder egyptian buffaloes. J. Anim. Health Prod. 9(4): 504-511.
DOI | http://dx.doi.org/10.17582/journal.jahp/2021/9.4.504.511
ISSN | 2308-2801
Copyright © 2021 Abdel-Khalek et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Low reproductive efficiency and longer calving interval are the major problems faced by buffalo breeders and farmers (Singh et al., 2000). In farm animals, the culling rate of repeat breeder animals causes economic losses, whereas it is an important disorder of animal reproduction. Repeat breeding was given in consideration when the female receive ≥3 insemination without pregnancy, and their genital tracts without obvious pathological disorders (El-Khadrawy et al., 2011). Disturbance in the hormonal balances and ovulation time, fertilization frailer, and early embryonic mortality are the main causes of repeat-breeding (Patel et al., 2014). In buffaloes, low conception rate in cows (Azawi et al., 2008) and heifers (Abo-Farw et al., 2009) is the main problem in the incidence of repeat breeding. Several attempts were carried out to reduce repeat breeding by administering hormone therapy to improve embryonic mortality (Singh et al., 2017). Some problems associated with repeat-breeders could be eliminated by the use of hormonal protocols designed to increase pregnancy rates (Rodrigues et al., 2010). In this respect, Abo-Farw et al. (2009) increase pregnancy rate of repeat breeder heifers to 70, 80 and 90% using GnRH- PGF2α-GnRH, GnRH at insemination and PGF2α-PGF2α (11-day interval), respectively. Also, pre-synchronization of estrus and ovulation using controlled internal drug release (CIDR) and PGF2α achieved improvement in pregnancy rate from 20 to 60% in buffalo-cows and from 0 to 60% in buffalo-heifers (Abo-Farw et al., 2019). It was mentioned that embryonic loss may be, partially, attributed to a decrease in progesterone (P4) secretion by corpus luteum (CL) during early pregnancy (Campanile et al., 2005) and elevated concentrations of circulating P4 are required for conceptus development in repeat breeding crossbred cattle (Savalia et al., 2014). More exposure to P4 by the embryo may increase its chance of secreting interferon-t and thus survive (Thatcher et al., 2001).
Lonergan et al. (2013) reported positive impacts on fertility by supplementing additional P4. In buffaloes, P4 is important for incidence and maintenance of pregnancy, and increasing embryo implantation rate (Kastelic, 1994). Many authors (Walsh et al., 2007; Chebel et al., 2010) reported improved reproductive efficiency of cyclic cows following treatment with different P4 devices. In this respect, using CIDR could be applied to improve fertility in buffaloes (Singh, 2003a) and repeat breeder Iraqi buffaloes (Azawi et al., 2012).
Progesterone is essential for the maintenance of pregnancy (Karen et al., 2014). Lesser concentrations of P4 may increase the risk of poor embryo quality after fertilization (Ahmad et al., 2004). Many methods have been tried to reduce embryonic mortality by enhancing endogenous P4 level as lower than the normal rise and lower total P4 concentration have been reported in repeat breeder cows (Gaja et al., 2008).
Based on the positive relationship between P4 post-insemination and the maintenance of pregnancy, the present study hypothesized that elevating post-mating period P4 level by CIDR may compromise conceptus development during pregnancy, which might increase the pregnancy rate in repeat-breeder buffaloes. So, the current study aimed to improve pregnancy rate by using CIDR at different intervals post-mating in repeat-breeder buffalo cows and buffalo heifers.
MATERIALS AND METHODS
This study was achieved by Animal Production Research Institute (APRI), Agricultural Research Center, Ministry of Agriculture, in cooperation with Animal Production Department, Mansoura University, Egypt. The experimental period lasted during the interval from May to September 2019. Animal Ethics Committee of Mansoura University, Egypt approved this study.
Animals
Total of 45 experimental animals with normal estrous cycles but failed to conceive after three successive services per animal (repeat breeder buffaloes) were taken from the herd of Mehallet Moussa Station of Animal Production, Kafrelsheikh Governorate, belonging to APRI. The experimental animals included 30 lactating Egyptian buffaloes three months in milk, weighing 450-500 kg, aging 4-5 years, and within 2-3 parities were used. Also, fifteen cyclic Egyptian buffalo heifers weighing 350-400 kg and aging 2.5-3 years were used in this study. The experimental buffalo cows showed normal calving and had no clinical illness signs detectable during the previous seasons. The rectal palpation and ultrasonography examination revealed intact reproductive tract of all experimental animals. Animals were housed under semi-open sheds. All animals were managed at similar feeding and managerial conditions applied in Animal Production Research Institute. Animals were free and kept under semi-open sheds.
Feeding system
The experimental animals were reared under traditional feeding and management systems; they were fed on diet that cover both maintenance and milk production requirements for buffaloes and based on LBW of heifers according to the recommendation of APRI. The ration of the experimental animals included concentrate feed mixture, CFM (33% un-decorticated cotton seed, 23% yellow corn, 22.3% wheat bran, 13% rice bran, 2% molasses, 3% limestone, 1.2% common salt and 2.5 % calcium), berseem hay (BH), corn silage (CS), and rice straw (CS). Fresh water was available all times. Table 1 show the chemical composition (on dry matter basis) of concentrate feed mixture, berseem hay, corn silage and rice straw. The methods of AOAC (2000) were used to obtain the chemical analysis of representative samples taken from all feedstuffs.
Experimental design
At the beginning of the experiment, thirty buffalo cows and fifteen buffalo heifers were divided into 3 groups (ten cows and five heifers each) based on live weight and age. Animals were naturally mated after estrous detection of the experimental animals (every morning and evening by visual observation twice daily, 6 a.m. and 6 p.m. for at least 30 min by teaser bull. Buffalo cows and heifers in heat were naturally mated by fertile buffalo bull.
On Day 5 post-mating, a CIDR-B device containing 1.9 g of progesterone (Vetrepharm Canada Inc., Belleville, ON, Canada) was inserted in the vagina of each animal for 4 days in the 1st group (Protocol 1, P1), and for 13 days in the 2nd group (Protocol 2, P2). CIDR was withdrawal on Day 9 post-mating in the 1st group and on Day 18 in the 2nd group. Animals in the 3rd group (Control) were left with
Table 1: Chemical composition of different feedstuffs (on DM basis).
| Feedstuff | DM % |
Chemical composition on DM basis (%) |
|||||
| OM | CP | CF | EE | NFE | ASH | ||
| CFM | 90.31 | 89.70 | 16.44 | 12.79 | 4.24 | 56.23 | 10.30 |
| BH | 88.52 | 88.45 | 15.32 | 23.43 | 5.14 | 44.56 | 11.55 |
| CS | 38.14 | 91.40 | 10.94 | 18.84 | 4.33 | 57.29 | 8.60 |
| RS | 91.02 | 83.06 | 2.70 | 36.93 | 1.53 | 41.90 |
16.94 |
CFM: concentrate feed mixture, BH: berseem hay, CS: corn silage, RS: rice straw, DM: dry matter, OM: organic matter, CP: crude protein, CF: crude fiber, EE: ether extract, NFE: nitrogen free extract.
out treatment during the experimental period at similar time near to other treatments. Blood samples were taken on Days 0 (estrus incidence), 3, 6, 9, 12, 15, 18, 21 and 24 post- mating, from all animals in each group. Pregnancy was diagnosed on day 24 post-mating by ultrasonographic examination. The experimental designs summarized in Figure 1.
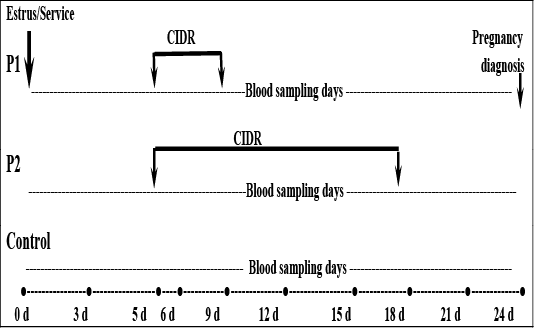
Figure 1: Diagram of the experimental design
Blood samples and progesterone assay
From the jugular vein of the experimental animals, blood samples were taken into clean test tubes without anticoagulants, left to clot, centrifuged (3000 rpm for 15 min) for serum collection which was stored in vials at -20oC until progesterone (P4) determination. The concentration of P4 was determined in blood serum using ready antibody coated tubes kit (Diagnosis Systems Laboratories Texas, USA) by direct Radioimmunoassay technique (RIA) after the methods of the procedures outlined by the manufacturer. A sensitivity of 0.073 ng/ml, and the inter- and intra-assay coefficients of variation, being 6.66 and 8.98%, respectively were recorded for P4 assay. The standard curve of P4 ranged from 0.1 to 30 ng /ml.
Diagnosis of pregnancy
After 24 days of mating each animals’ pregnancy was ultrasonographically diagnosed (Digital ultrasonic diagnostic imaging System, Model Dp-30 Vet. 50/60 HZ, SHENZHEN, MINDRAY BIO-MEDICAL.ELECTRONICS, CO. LTD) using 7.5 MHz Linear array transducer at a depth of 4.3 cm. On day 45-55 post-mating, pregnancy was rectally palpated to insure non-returned to estrus animals, then pregnancy rate was computed.
Statistical analysis
To test the differences in P4 concentration among groups (protocol 1, protocol 2 and control), one way-ANOVA design was applied by using software package SAS program (SAS, 2004). The differences in P4 concentration between pregnant and non-pregnant animals were determined by T-test student analysis. The significant differences among groups were performed by Duncan Multiple Rang test (Duncan, 1955) at P < 0.05. within SAS program. Pregnancy rate of buffaloes was compared using χ2 test.
RESULTS
Pregnancy rate
In the current study, results in Table 2 revealed that pregnancy rate (PR) of cows, heifers, or as overall was higher (P< 0.05) in both CIDR groups than control, but PR was higher (P< 0.05) in protocol 2 (P2) than in protocol 1 (P1). Pregnancy rate was higher (P < 0.05) for cows than heifers, regardless of treatment (Table 2).
Overall P4 profile during post-mating period
CIDR treatments vs. controls
Results of P4 profile at estrus and different post-mating days revealed insignificant differences in P4 concentration between CIDR groups and controls on Day 0 and 3 post-mating (before CIDR treatment). Concentration of P4 in P1 and P2 was higher (P< 0.05) than in the control group, following CIDR incursion for both protocols, on Days 6, 9 and 12 post-mating. From Day 15 up to 24 post-mating, P4 concentration markedly increased (P< 0.05) in P2 than in P1, while the controls showed the lowest P4 values. Concentration of P4 increased (P< 0.05) from Day 6 to 9 post-mating in P1, and from Day 6 to 18 post-mating in P2, but showed insignificant change in the controls during different post-mating days (Table 3).
Buffalo cows vs. buffalo heifers
Concentration of serum P4 showed similar trend of change in cows and heifers from estrus up to Day 24 post-mating.
Table 2: Effect of CIDR treatment on pregnancy rate of repeat breeder buffalo cows and heifers.
| Item |
P1 (n= 15) |
P2 (n= 15) |
Control (n= 15) |
Total |
|
Buffalo cows (n=30) |
5/10 (50%)b |
7/10 (70%)a |
2/10 (20%)c |
14/30 46.7%)A |
| Buffalo heifers (n=15) |
2/5 (40%)b |
3/5 (60%)a |
0/5 (0%)c |
5/15 (33.3%) B |
| Overall (n=45) |
7/15 (46.7%)b |
10/15 (66.7%)a |
2/15 (13.3%) c |
19/45 (42.2%) |
a,b,c Means with different small superscripts in the same row are significantly different (P< 0.05)
A,B Means with different capital superscripts in the same column are significantly different (P< 0.05)
Table 3: Serum P4 concentration (ng/ml) of animals in the control and CIDR groups (P1 and P2) at estrus and successive post-mating days.
| Post-mating day |
P1 (n= 15) |
P2 (n= 15) |
Control (n= 15) |
P value |
|
0 |
0.46±0.021 | 0.43±0.021 | 0.46±0.021 |
0.7696ns |
| 3 | 0.55±0.019 | 0.59±0.030 | 0.62±0.031 |
0.3691ns |
| 6 |
1.11±0.088 a |
1.16±0.089 a |
0.73±0.034b |
0.0004*** |
| 9 |
1.57±0.201 a |
1.43±0.120 a |
0.81±0.075b |
0.0025** |
| 12 |
1.49±0.149 a |
1.63±0.156 a |
0.95±0.078b |
0.0046** |
| 15 |
1.59±0.207b |
2.11±0.222 a |
0.93±0.093c |
0.0008*** |
| 18 |
1.72±0.288b |
2.66±0.034 a |
0.89±0.127c |
0.0006*** |
| 21 |
1.78±0.318 b |
2.52±0.332a |
0.94±0.151c |
0.0027** |
| 24 |
1.95±0.371b |
2.63±0.373a |
0.73±0.219c |
0.0016** |
a-c Means with different superscripts in the same row are significantly different P< 0.05. ns: not significant; **:significant at P<0.01; ***:significant at P<0.001
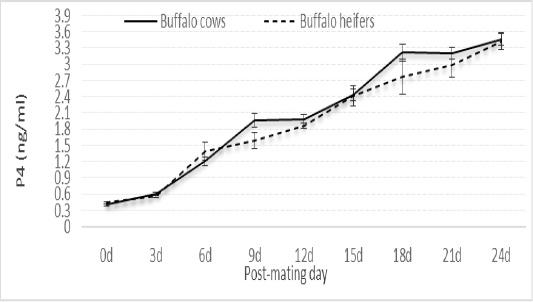
Concentration of P4 at estrus was higher in heifers than in cows, nearly similar on Day 3 post-mating, and higher in cows than in heifers up to Day 24 post-mating, but all differences between heifers and cows were not significant (Figure 2).
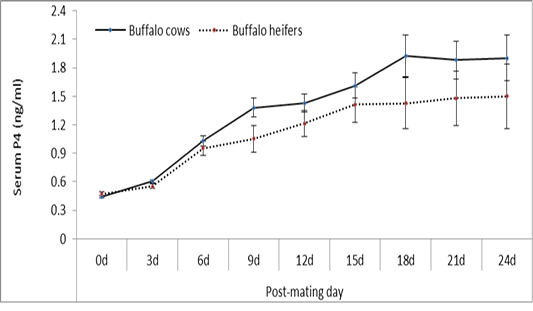
Figure 2: Changes in serum P4 concentration at estrus and successive post-mating days of buffalo cows (n= 30) and heifers (n= 15).
Profile of P4 in pregnant and non-pregnant animals
Concentration of P4 at estrus was lower (P < 0.01) in pregnant than in non-pregnant. On Day 3 post-mating the differences in P4 concentration between the pregnant and non-pregnant animals were not significant, thereafter P4 concentration increased (P < 0.001) in pregnant as compared to non-pregnant animals (Figure 3). This trend was observed between pregnant and non-pregnant animals in P1, P2 and control, as well as in cows and heifers.
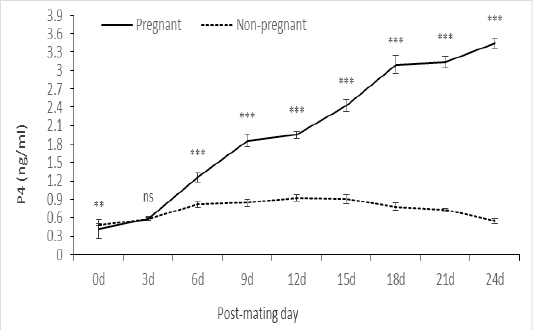 Figure 3: Serum P4 level at estrus and post-mating of pregnant (n= 19) and non-pregnant (n= 26) animals. ns: not significant; **: significant at P<0.01; ***: significant at P<0.001
Figure 3: Serum P4 level at estrus and post-mating of pregnant (n= 19) and non-pregnant (n= 26) animals. ns: not significant; **: significant at P<0.01; ***: significant at P<0.001
Profile of P4 in pregnant animals
CIDR treatments vs. controls
Results in Figure 4 revealed non-significant differences in P4 concentration as affected by CIDR treatments up to Day 6 post-mating. On Day 9 up to 24 post-mating, P4 concentration was higher (P < 0.01) in CIDR treatments than controls, being the highest in P2 than in P1 on most post-mating days.
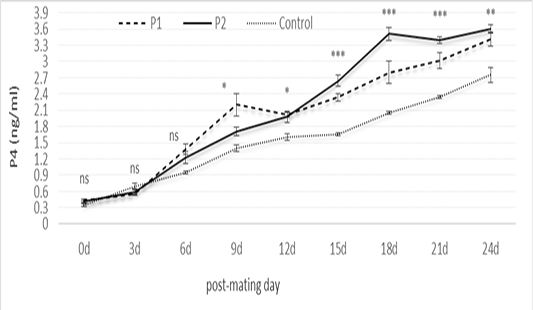 Figure 4: Serum P4 level at estrus and post-mating of pregnant animals in P1 (n= 7), P2 (n= 10), and controls (n= 2). ns: not significant; *: significant at P<0.05; **: significant at P<0.01; ***: significant at P<0.001
Figure 4: Serum P4 level at estrus and post-mating of pregnant animals in P1 (n= 7), P2 (n= 10), and controls (n= 2). ns: not significant; *: significant at P<0.05; **: significant at P<0.01; ***: significant at P<0.001
In pregnant buffalo cows and heifers
The trend of change in P4 concentration on different post-mating days was similar in buffaloes and heifers. On all post-mating days, P4 concentration was nearly similar at estrus and 3 days post-mating, and higher (P≥ 0.05) in buffaloes compared with heifers on other post-mating days (Figure 5).
DISCUSSION
The current study aimed to improve pregnancy rate of repeat-breeder Egyptian buffaloes by using CIDR treatment at different intervals post-mating. In repeat breeder dairy-cows, various CIDR protocols could be used to improve pregnancy rate (Ghasemzaeh-Nava et al., 2007) and improve the fertility of repeat breeder Iraqi buffaloes (Azawi et al., 2012). To avoid long calving intervals, culling rate, and decrease of milk production due to subjecting buffaloes to ≥3 services/animal, animals in our study were administrated with supplemental P4 (CIDR) at two different intervals post-mating of P1 (day 5-9) and P2 (day 5-18) in repeat breeder buffalo cows and heifers. In our study, all experimental buffalo cows were without reproductive disorders (dystocia, retained placenta, endometritis) at the previous season, and buffalo heifers were healthy with proper LBW and cyclic, but all experimental animals failed to conceived after three conductive mating cases.
In our study, we improved overall PR of cows and heifers from 13.3% (2/15) in repeat breeder Egyptian buffaloes to 56.7% (17/30), being higher (P<0.05) in P2 than P1 (66.7 vs. 46.7%) and in cows than heifers (46.7 vs. 33.3%). In bovine, Mehni et al. (2012) reported that Holstein cows treated with CIDR showed greater PR as compared to untreated animals (56 vs. 25%). Also, CIDR treatment improved PR of dairy cattle from 35 to 48% (Sandra et al. 2007). CIDR treatment on Day 5-12 after insemination was an effective method to improve fertility in repeat breeder cows (Reza et al., 2012). Contrary, Shams-Esfanabad and Shirazi (2006) found that CIDR incursion from 5-19 days after insemination did not improve PR of repeat-breeder cows. In another way, Abo-Farw et al. (2016) in Egyptian buffalo heifers and Attoo et al. (2013) in Indian buffaloes found that PR significantly improved when animals were treated with GnRH on 13-day post-mating as compared to those treated on 0 or 11-day post-mating. PR significantly increased in buffaloes treated with CIDR as compared to controls (40 vs. 0%, Azawi et al., 2012).
In domestic animals, the formation of complete CL with capacity of progesterone secretion is indicator of early pregnancy (Bulman and Lamming, 1978). Increasing P4 level improve the ability of interferon-τ (IFN) production for pregnancy regulation (Binelli et al., 2001). Also, P4 was reported to suppress the apoptosis in the luteal cells of bovine (Okuda et al., 2004). These findings indicated the possibility of PR improvement by increasing plasma P4 concentrations (Bó et al., 2002). In this respect, Van Cleeff et al. (1996) suggested that increasing P4 concentration could overcome the insensitivity of P4 receptors which may lead to increased PR. In accordance with improving PR in CIDR protocols, Mehni et al. (2012) found that the mean P4 concentration in dairy cattle after CIDR insertion (Day 5-19 after AI) was higher than untreated group. It is of interest to note that P4 concentration of both buffalo cows and heifers was ≥1 ng/ml in P1 and P2 on day 6 post-mating (after CIDR insertion). However, P4 level was < 1 ng/ml in control buffaloes. Colazo et al. (2004) and Muth-Spurlock et al. (2016) mentioned that the surge release of LH and estrus incidence need blood P4 concentration of ≥ 1 ng/ml to avoid the inhibitory mechanism of CIDR device administration. In similarity with the present study, Sandra et al. (2007) observed that the circulating P4 concentrations increased by 0.7 ng⁄ ml in CIDR treatment after AI in comparing the controls.
Many authors suggested that the rise of post-mating P4 level also by GnRH, leads to improved PR. In this context, GnRH analogue injection on day 13 post-mating in repeat breeder buffalo cows (Abo-Farw and Ghoneim, 2017) may stimulate the transformation of follicular cells to luteal cells, which was required at least 2 to 3 days for optimum P4 production (Stevenson et al., 1993). Also, Nussara et al. (2008) indicated that the administration of exogenous GnRH in luteal phase post inseminations in dairy cattle resulted in increased serum P4 concentration. This might be beneficial in reducing early embryonic mortality especially in infertile or heat-stressed or repeat breeding cows which might have less P4 concentration after insemination. These findings may indicate the positive effect of CIDR on elevating P4 level in CIDR treatment groups as compared to control ones on different post-mating days. In this respect, Chacher et al. (2017) found an association of blood P4 concentration with conception rate in lactating cows and heifers. In our study, P4 level increased in cows than in heifers on different post-mating days, regardless of CIDR treatment, but the differences in P4 between cows and heifers were not significant. In this way, Takkar et al. (1982) found that the mean P4 concentration of different days of the cycle did not differ significantly between heifers and cows.
In accordance with the P4 profile on post-mating days for pregnant and non-pregnant animals in our study, Abo-Farw and Ghoneim (2017) reported that overall mean of P4 concentration on Day 23 and 25 post-mating was lower in non-pregnant (0.785±0.158 and 1.314±0.123 ng/ml) than in pregnant (6.679±0.218 and 6.381±0.247 ng/ml) buffaloes. Also, Raj et al. (2016) recorded a greater number of buffaloes became pregnant when P4 concentrations at the time of AI was below the suprabasal level and the pregnancy reduced when the P4 level is between suprabasal and 1 ng/ml. None of the cows become pregnant when the P4 level was more than 1 ng/ml. Mean values of serum P4 at estrus were 0.895±0.134 ng/ml in pregnant and 1.429±0.235 ng/ml in non-pregnant buffaloes. The lowest PR and P4 of controls presented in this study, particularly for buffalo-heifers, may suggest that increased serum P4 level may rise the PR, regardless of CIDR treatment (Mehni et al., 2012). Our results indicated that PR was positively affected by CIDR-treatments having the greatest effect on P4 level on different post-mating days. The CIDR treatment markedly affects serum P4 level and PR, whereas the administration of CIDR during days post-mating (day 5) increased serum P4 level in buffalo cows and heifers.
Generally, P4 is an essential hormone in the maintenance of pregnancy in cows. The higher P4 levels in the early pregnancy are related to the embryonic development and increase in interferon-τ production and PR (Beltman et al., 2009). Animals on day 5, with higher plasma P4 concentrations, had developmentally more advanced and viable embryos (Green et al., 2010). In this context, Leyan et al. (2013) indicated that the benefit of P4 supplementation on the fertility of cows required exogenous P4 supplementation to start between day 3-7 and the appropriate reproductive status of the treated cows. These findings support the observed higher P4 level after CIDR insertion in pregnant than non-pregnant in P1 and P2 in comparing with control, being higher in P2 than in P1. Also, P4 level was higher in pregnant animals in P2 than in P1, being higher than in pregnant control animals. In our study CIDR insertion from Day 5 to 9 post-mating resulted in lower PR than in those treated with CIDR (Day 5-18). However, CIDR treatment on day 5 until 12 after insemination was effective method to improve fertility in repeat breeder cows due to a direct effect of P4 on fetal development (Reza et al., 2012) and embryo viability (Leroy et al., 2008). The CIDR device improved PR by reducing embryonic losses in dairy heifers (Alnimer, 2009).
Generally, results of this study support the hypothesis that a CIDR device when properly used, on Day 5 to 18 post-mating increased P4 level in the majority of repeat breeder buffaloes within few days after CIDR removal and reduced embryonic losses. Green et al. (2010) discussed the positive effects of P4 on reducing embryonic mortality, stating that P4 concentration on Day 4 and 5 has a positive correlation with interferon-tau (IFN-s) production on Day 16 post-AI. Similarly, Mann and Lamming (2006) reported that P4 treatment at the interval from Day 5 to 9 post-AI increased the production of IFN-s by the embryo. Finally, Leroy et al. (2008) mentioned that early embryonic life is incapable of sub-optimal uterine micro-environment and inadequate CL function with reduced P4.
CONCLUSION
According to the obtained results, CIDR treatment of repeat breeder buffaloes on day 5 post-mating that was removed on day 9 (P1) or on day 18 (P2) had benefited from progesterone supplementation for improvement of pregnancy. However, long P4 treatment (P2) is concluded for buffaloes suffering from low P4 level at the early pregnancy stage and repeat breeding. Increasing P4 level by CIDR treatment from 5-18 days post-mating could be recommended as a useful strategy to reduce the embryonic mortality and improving pregnancy of repeat breeder buffalo cows and heifers.
CONFLICT OF INTEREST
There are no conflict of interests of this manuscript.
AUTHOR CONTRIBUTIONS
Aim and design of the manuscript (A.E. Abdel-Khalek and M.A. Abo-Farw), acquisition of data (M.A. Abo-Farw and W. M. Nagy), statistical analyses of data (A.E. Abdel-Khalek and M.A. Abo-Farw), statistical analyses (M.A. Abo-Farw and A.E. Abdel-Khalek), drafting the manuscript (M.A. Abo-Farw, A.E. Abdel-Khalek and W.M. Nagy), revision of the manuscript (A.E. Abdel-Khalek and M. A. Abo- Farw), and preparing the manuscript for publication (all authors).
REFERENCES