Journal of Animal Health and Production
Research Article
Protective Effect of Edible Bird’s Nest on Ovaries of Cycling Female Rats Subjected to Cadmium Toxicity
Abdul Quddus 1,4, Nurhusien Yimer1*, Faez Firdaus Abdullah Jesse1,M. Mustapha Noordin 2, Mark W. H. Hiew1, Muhammad Abdul Basit3, Maria Amir1, Mohammed Sirajul Islam1,6, Ubedullah Kaka4
1Department of Veterinary Clinical Studies, Faculty of Veterinary Medicine, University Putra Malaysia, 43400 Serdang, Selangor, Malaysia; 2Department of Veterinary Pathology & Microbiology, Faculty of Veterinary Medicine, Universiti Putra Malaysia, 43400 UPM, Serdang; 3Department of Preclinical Sciences, Faculty Veterinary Medicine, Universiti Putra Malaysia, 43400 UPM, Serdang; 4Department of Companion Animal Medicine & Surgery, Faculty Veterinary Medicine, Universiti Putra Malaysia, 43400 UPM, Serdang; 5Faculty of Veterinary and Animal Science, the Lasbela University of Agriculture Water and Marine Science, Uthal, Balochistan, 90150, Pakistan; 6Bangladesh Livestock Research Institute, Savar, Dhaka, Bangladesh.
Abstract | Cadmium (Cd), a toxic heavy metal, is often associated with the reproductive disorders of mammals. The edible bird’s nest (EBN) is a naturally occurring food product made from the saliva of swiftlet birds (Aerodramus fuciphagus & Aerodramus maximus) in the form of a nest, and it has been consumed as healthy food or tonic for decades. This research aimed to study the possible protective effects of EBN against Cd toxicity in ovaries of Sprague Dawley rats. Thirty (30) female Sprague Dawley rats were assigned into five groups as follows: group 1-Negative control (NC) received distilled water; group 2-positive control (PC) administered with CdCl2, 5mg/kg BW; while groups EBN 1, EBN 2, and EBN 3 received CdCl2 (5mg/kg BW) concurrently with graded concentrations of 60, 90 and 120 mg/kg BW of EBN, respectively. After four weeks of treatment, rats were euthanized to collect ovaries for histopathological studies using hematoxylin and eosin (H&E) stain. Expressions of vascular endothelial growth factor (VEGF) were analyzed through immunohistochemistry, and levels of superoxidase dismutase (SOD) were assessed using a SOD assay kit. Oral administration of CdCl2 without EBN supplement resulted in significantly decreased (p<0.05) immunohistochemical expressions of VEGF in ovarian tissue and a decrease (p<0.05) in the activity of SOD. Moreover, ovarian histopathological changes, including follicular cysts and a significant increase (p<0.05) in the number of atretic follicles, while a decrease in the number of growing follicles was noted in Cd only treated group. Animals treated with CdCl2 and EBN at three different dosages resulted in significantly increased (p<0.05) expressions of VEGF in ovarian tissues, low degenerative changes with normal histomorphology as well as significantly increased (p<0.05) SOD activity as compared to the PC group. Overall, the findings revealed that oral exposure to Cd at a dose rate of 5 mg/kg BW resulted in significant alterations in ovaries, as evidenced by a lower degree of VEGF expression along with reduced antioxidant activity and histomorphological changes. Meanwhile, EBN proved to exhibit a significant protective role against Cd toxicity in ovaries, possibly through its antioxidant effect.
Keywords | Cadmium toxicity, Cycling female rats, Edible Birds Nest, Ovary
Received | June 07, 2021; Accepted | June 20, 2021; Published | November 15, 2021
*Correspondence | Nurhusien Yimer, Department of Veterinary Clinical Studies, Faculty of Veterinary Medicine, University Putra Malaysia, 43400 Serdang, Selangor, Malaysia; Email: nurdeg2006@gmail.com
Citation | Quddus A, Yimer N, Jesse FFA, Noordin MM, Hiew MWH, Basit MA, Amir M, Islam MS, Kaka U (2021). Protective effect of edible bird’s nest on ovaries of cycling female rats subjected to cadmium toxicity. J. Anim. Health Prod. 9(4): 496-503.
DOI | http://dx.doi.org/10.17582/journal.jahp/2021/9.4.496.503
ISSN | 2308-2801
Copyright © 2021 Yimer et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Cd is one of the most harmful and widespread environmental and occupational pollutants in industrialized countries (Järup et al., 2015; Lane et al., 2015). It has been classified among ten (10) highly toxic compounds for human health (Joseph, 2009). Cd is extensively present in the environment; advances in civilization processes and industrialization have doubled the rise in Cd level in the environment, consequently elevating its presence in the food chains and risking human and animal health (WHO, 2010). Cd is present in PVC pipes, phosphatic fertilizers, and color pigments, with few Cd-containing products recycled (Järup & Åkesson, 2009). Cigarette smoke and food are the most critical sources of Cd exposure in the human population (Piasek et al., 2001), while animals are exposed through several other sources (Zhang & Reynolds, 2019). It can enter the body via daily eating, which contains a considerable amount of Cd in normal conditions (Ismahil, 2007). Cd has an exceptional biological half-life, around 30 years in an organism which highlights the need for treatment of its toxicity (Brzóska et al., 2016). Many studies have outlined the effects of Cd on spermatogenesis and oogenesis in females and males, respectively, and implicating its compounds in early implantation failure and embryo lethality (Thompson & Bannigan, 2008). Various experimental studies on female rats revealed that with a considerably quite long half-life, it accumulates in the reproductive tract (Nasiadek et al., 2011). It has caused endometriosis in female rats (Nasiadek et al., 2018). Moreover, uterine length and the number of uterine implantation sites were decreased due to Cd exposure (Henson & Chedrese, 2004). Furthermore, Cd can alter ovarian functions, inhibit the development and growth of follicles, increase the percentage of follicular atresia, cause ovulation failure, malfunctioning of implantation, and congenital disabilities (Thompson & Bannigan, 2008).
In recent decades, a great deal of concern has been given to the biochemical roles of natural substances in biological systems (Brzóska et al., 2016). Various plant and animal origin compounds are reported to show a protecting role against heavy metal toxicity (Dailiah Roopha & Padmalatha, 2012). Edible bird’s nest (EBN), the nest made from the salivary secretions of swiftlet birds, has been consumed as healthy food or tonic for decades. It has shown strong medicinal benefits in enhancing the immune system, stimulating epidermal growth factor, inhibiting viral infections in mice and rats (Albishtue et al., 2018a; Daud et al., 2019; Haghani et al., 2016). A study conducted on female rats demonstrated that EBN increases the fertility rate and the rate of embryo implantation by enhancing the differentiation and proliferation of ovarian structures, as demonstrated by up-regulation of steroid receptor expressions (Albishtue et al., 2018b). As of today, there is a lack of information on Cd-mediated reproductive toxicity in female rats. Based on all these observations, the current study was designed to examine whether EBN has an ameliorating effect on Cd exposure in the ovary of an experimental rat model.
Material and Methods
Laboratory Animals
This study was carried out at the Faculty of Veterinary Medicine, Universiti Putra Malaysia (UPM). Thirty Sprague Dawley rats (12 weeks old) were collected from the Animal resource unit, UPM. Animals were acclimatized for one week, allowing ad libitum access to rodent diet and water. The rats were kept at standard humidity & temperature and 12/12-h light/dark cycles. Animals were kept in compliance with the Institutional Animal Care and Use Committee guidelines, Universiti Putra Malaysia (UPM) (Project approval number: UPM/IAUC/AUPR-009/2016).
Preparation of EBN extract
EBN was obtained from Agridon technologies, Malaysia (House bird’s nest), and stored at 25°C-32°C. EBN solution was made as per the Chinese custom of making bird’s nest soup. The nests were cleaned by soaking in water, after softened, inspected for any impurity. The clean nests were then air-dried at ambient temperature and powdered with an automatic mixer (BUCHI.400, Switzerland), the EBN powder was kept at 4 ° C, later, EBN solution was made by dissolving 1 gram of EBN powder in 100 mL of distilled water and heated at 60 ° C for 45 minutes in a water bath. Eventually, The EBN solution was kept at room temperature for a while and then administered to the rats at different doses based on the BWs.
Preparation of Cd Solution
Cadmium Chloride with a molecular formula CdCl2 and molecular weight (183.32g/mol) was purchased from R&K Chemicals, UK. CdCl2 was readily dissolved in distilled water and administered via oral gavage for four weeks (Nna et al., 2017).
Experimental design
As shown in Table 1, rats were divided randomly into five groups (six animals/group) as follows: group 1-negative control (NC) received distilled water (1ml); group 2-positive control (PC) administered with CdCl2 (5mg/kg BW); while group EBN 1, EBN 2 and EBN 3 received CdCl2 (5mg/kg BW) concurrently with concentrations of 60, 90 and 120 mg/kg BW of EBN, respectively. CdCl2 dosages were in accordance with Nna et al. (2017), while EBN doses were chosen, according to Albishtue et al. (2019). The rat’s BW was measured and recorded weekly in each group during the administration period. After 30 days of exposure, rats found in estrus were weighed and euthanized following the general anesthesia protocol. General anesthesia comprised injections of 30 mg ketamine/kg BW & 10 mg xylazine/kg BW to collect blood (Ruslee et al., 2020).
| Groups | Names assigned | Type of feed and dose |
| Control | NC | ND + distilled water (1ml) |
| Treated | PC |
ND + CdCl2 (5mg/kg BW) |
| EBN 1 |
ND +EBN (60mg/kg) + CdCl2 (5mg/kg BW) |
|
| EBN 2 |
ND +EBN (90mg/kg) + CdCl2 (5mg/kg BW) |
|
| EBN 3 |
ND +EBN (120mg/kg) + CdCl2 (5mg/kg BW) |
NC= Negative control, PC= Positive control, ND=Normal diet, CdCl2=Cadmium Chloride, EBN=Edible bird’s nest,
Estrous cycle determination and synchronization
The rats were synchronized at the beginning of the experiment, with two 0.5 mg of Estrumate intraperitoneal doses per rat, three days apart. Vaginal smears were obtained every morning for 30 days from all animals to identify regular 4-day estrous cycles, and cytological analysis was performed by light microscopy according to Nasiadek et al. (2019).
Macroscopic examination and organ weight
Ovaries were harvested, cleared of any connective tissue, washed with normal saline solution, and weighed using an electronic weighing balance machine (Adam Equipment Inc, Oxford). Any visible abnormalities found during the gross examinations were noted. Ovarian body weight ratio (OBWR) was measured according to the following equation: [ovarian weight (g)/body weight (g)] X100.
Histopathological examination
Ovaries were placed in a 10% formalin solution immediately, then embedded into paraffin blocks, cut into 5-μm pieces, and stained by using hematoxylin-eosin (H&E) for histopathological examination. Histopathological examination was performed under a Medical imaging microscope (Motic Image plus 2.0, China) attached with a camera, and representative photomicrographs were captured. Ovarian follicles were counted in all rats using image analyzer software (Motic image software Plus 2.0, China). The follicles were quantified at x400 in ovaries from three rats in each group. The proportion of each stage follicles per ovary was determined by counting the total number in five representative sections per ovary. Follicles were categorized according to antral space possession and the number of layers surrounding the oocyte: primordial follicle, primary follicle, secondary follicle, tertiary or antral follicle, and atretic follicle (Luo et al., 2008).
Analysis of the expressions of VEGF in the ovaries by Immunohistochemistry
VEGF expressions were analyzed by using the Immunohistochemistry technique according to the protocol described by Albishtue et al. (2019), collected sectioned (4 μm) tissue on clean glass slides from paraffin-embedded blocks and allowed to air dry for 15 hours. Slides were deparaffinized with a series of xylene and alcohol. For antigen retrieval, sections were immersed in a container filled with diluted Target Retrieval Solution (pH 6.0) (Dako, North America). The container was placed in a microwave for 10-minute-high power, then cooling naturally to ambient temperature. Target Retrieval Solution was decanted, and sections were rinsed three times with tris buffered solution (TBS). Removed the excess wash buffer by tapping, and the Dako pen (Dako, Denmark) was used to mark the tissue. A peroxidase block solution, IHC detection kit (Code ab236469, Abcam USA), was applied to the specimen and incubated at 37 ° C for 10 min to inactivate the endogenous peroxidase. Samples were rinsed with TBS. To prevent endogenous immunoglobulins from reacting with the secondary antibody, the protein block was applied to the specimen and incubated at 37° C for 10 min and bathed with TBS. Then, the specimens were incubated with rabbit polyclonal antibody against VEGF (Anti-VEGF monoclonal antibodies, Abcam USA ab192385, 1:150) from Abcam, USA for 30 min at 4° C. For the negative control, another section was prepared without the inclusion of primary antibodies. The specimens were rinsed with TBS and incubated with the Peroxidase-labelled polymer secondary antibody, IHC detection kit (Code ab236469, Abcam USA) at 37° C for 15 min. Slides were developed with chromogen DAB (3, 30-diaminobenzidine) for 10 min. Rinsed with distill water to stop the staining. For counterstaining, immersed the slides in a bath of hematoxylin for 3 min. Immuno-expression was analyzed using electronic images taken with a microscope (Leica Microsystems, Germany) attached to a digital high-resolution video camera (Leica, Germany) and a color monitor (Acer, Taiwan). VEGF expression was assessed for immunostaining intensity in the various cells and graded based on the following four grades: 0: absent; 1: weak positive; 2: moderate; 3: strong. The scoring method mentioned above was adopted from Godbole et al. (2007) with slight modification.
Assessment of antioxidant and oxidative stress biomarker assay
Enzymatic antioxidants activity, SOD, was assessed in blood plasma using a SOD assay kit (E-BC-K020, Elabscience, USA).
Statistical methods
All findings are shown as a mean (M) ± standard error mean (SEM) and analyzed with Graph Pad Prism 8.0 (Graph Pad Software, San Diego). One-way analysis of variance (ANOVA) followed by Tukey multiple comparisons posthoc test was used to compare the number of follicles, ovarian body weight ratio, expression levels of VEGF, and SOD activity in blood plasma. ANOVA, through Bonferroni’s multiple comparison tests, was applied for body weight. A P value < 0.05, suggesting a statistically significant difference, was considered.
Results and discussion
Body and ovary weight
Cd toxicity impaired the growth of rats, and the average weight of the PC group was significantly lower (p<0.05) than that of the NC group and EBN treated groups. Among EBN treated groups, EBN 3 was not significantly different (p>0.05) from the NC group (Figure 1). Moreover, the OBWR was significantly lower (p<0.05) in the PC group as compared to NC and EBN treated groups (Figure 2). The present study demonstrated that four weeks of CdCl2 exposure (5 mg/kg BW) in the absence of EBN supplement adversely affected body weight and OBWR; Cd at a lower dose has shown an adverse effect on the body weight of female rats, as demonstrated by previous studies (Nampoothiri et al., 2007; Nna et al., 2017; Samuel et al., 2011). A study conducted by Sajjad et al. (2014) had found a relationship between body weight loss and concentration of Cd in hypothalamus and pituitary of Cd exposed animals. The loss in body weight due to Cd exposure may be associated with the formation of free radicals resulting in oxidative stress (Patra et al., 2011). EBN treatment protected against Cd-induced wasting and bodyweight reduction in all the EBN treated rats, evidenced by significantly higher body weight gains compared to the PC Group (Figure 1). This effect of EBN may be attributed to its strong antioxidant activity and high nutritional value (Dai et al., 2020).
Histopathological examination
Histological sections of the ovaries from the NC group depicted well-developed ovarian follicles at a different stage of ovarian development and corpus luteum (CL) with normal stromal cells and blood vessels. In comparison, the ovaries of the PC group presented atrophied ovaries accompanied by intense follicular atresia and the formation
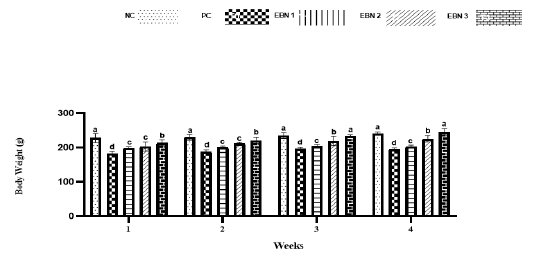
Figure 1: Effect of EBN on the body weight of rats. Data are presented as means ± standard error (SE). Different letters a, b, c within rows denotes significant difference at p < 0.05.
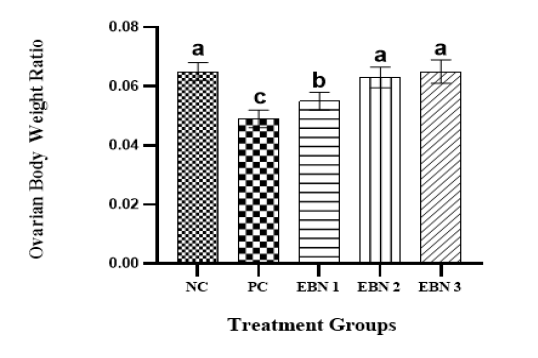
Figure 2: Effect of EBN and CdCl2 on Ovarian - body weight ratio. Values are expressed as Mean ± SEM. Bar graphs with different alphabets show significant differences at P< 0.05.
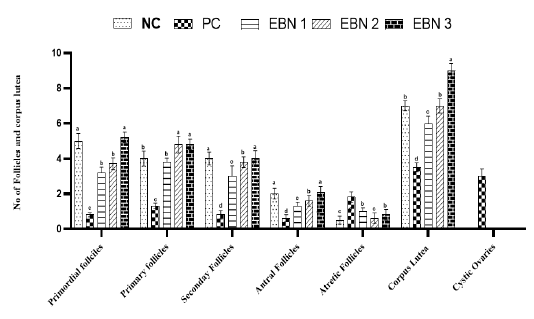
Figure 3: Effect of EBN on development of follicles after Cd exposure in female rats. Data are presented as Mean± SEM. Different letters a, b, c, and d exhibit significant differences at p <0.05. Note. Increase in no of growing follicles in negative control and EBN treated groups as compared to PC group (Cd only treated group). A significant rise in the number of cystic follicles is evident in the PC group.
of follicular cysts (Figure 4). These follicular cysts showed a thin granulosa layer and large antrum. EBN showed protection against the pathological changes brought by Cd, EBN treated groups demonstrated well development of ovarian follicles at different stages with significantly lower (p<0.05) number of atretic follicles. The number of surviving follicles and CL were significantly higher (p<0.05) at the EBN dose of 90mg/kg and 120mg/kg (Figure 3). Previous animal studies have shown that Cd exposure may alter ovary morphogenesis and cause a decrease in the number of growing follicles and an increase in follicular atresia (Cheng et al., 2019; Nna et al., 2017; Saedi et al. 2020). The mitigation of primordial follicles can lead to permanent infertility due to the ultimate reduction of the pool of the follicles (Nad et al., 2007). Animals supplemented with EBN showed protection against degenerative changes by exhibiting a significant increase in the number of surviving follicles & CL and a decrease in the number of atretic follicles.
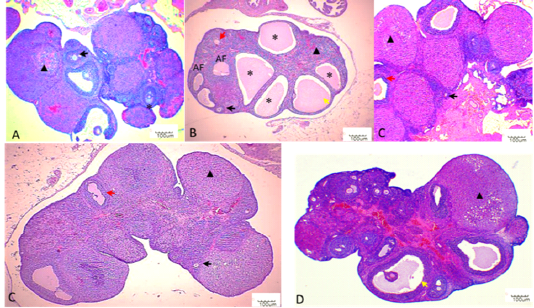
Figure 4: Histopathological findings in ovaries. Photomicrographs were taken at 40X. Black arrows indicate primary follicle, and red arrows indicate secondary follicle, yellow arrow indicate antral follicle, asterisk indicate ovarian cyst, black triangle indicates corpus luteum. A: NC; B: PC; C: EBN 1; D: EBN 2, and E: EBN 3.
Similarly, EBN has previously shown beneficial effects on the maturity and development of the follicles and increases the number of growing follicles (Albishtue et al., 2018a). This effect of EBN might be due to its potential bioactive components; the nutritional composition of EBN is protein, carbohydrate, ash, and lipids (Ma & Liu, 2012), while sialic acid is the major carbohydrate component found in EBN (Daud et al., 2019). Sialic acid-containing gangliosides have been demonstrated to efficiently protect oocytes and embryos from ROS produced injury, which is one of the major mechanisms of Cd toxicity (Kim et al., 2020; Wang et al., 2019).
Immunohistochemical expression of VEGF in ovarian tissues
Representative immunohistochemistry photomicrographs with VEGF expressions are shown in the ovarian sections of all groups (Figure 5). The results revealed that the VEGF gene was expressed primarily in the interstitial cells and surface epithelium. The increased dose of EBN enhanced the rate of expression of VEGF in the interstitial cells and ovarian surface epithelium (Figure 5)). Therefore, the increase in expression was observed in all the above parameters in the higher dose of EBN (120mg/kg BW) as compared to group NC and other treated groups (p < 0.05). The expression levels in EBN treated groups were significantly higher than those in the PC group. Alterations in VEGF expression are linked to disturbance of the ovarian and uterine functions (Hervé et al., 2006). This statement is confirmed by histopathological findings of the current study, in which decreased number of growing follicles was evidenced due to Cd exposure. EBN treated groups showed significantly higher immunohistochemical expression of VEGF in ovarian epithelial and interstitial cells; this finding is consistent with the study by (Albishtue et al., 2018b) in which EBN supplement increased expression levels of VEGF in ovaries. A study conducted by (Irusta et al., 2010) showed that VEGF promotes proliferation of follicular cells in rats and prevents apoptosis of early antral ovarian follicles, further it was confirmed that VEGF could act on follicular cells along with E2 and FSH through stimulating proliferation and prevention of apoptosis, thus promoting follicular growth and selection of the specific follicle to ovulate (Nash et al., 2006). This is in agreement with the current study in which histopathological findings revealed a significantly increased number of surviving follicles and a decrease in atretic follicles in ovaries of EBN supplemented groups. It has been confirmed that EBN comprises VEGF and IL-6, which averts the apoptosis of embryonic neurons by inhibiting the stimulation of caspase III, leading to the suppression of apoptotic cells (Roh et al., 2012). Thus, Increased levels of VEGF provide further confirmation of the protective role of EBN.
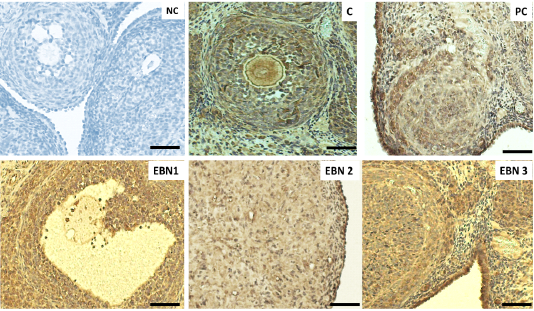
Figure 5: Immunohistochemical expressions of VEGF in ovaries. Photomicrograph sections of ovaries different experimental groups treated with CdCl2 and different doses of EBN showing VEGF expression. A remarkably higher VEGF expression on the ovarian surface and interstitial cells are visible in negative control, and EBN treated groups as compared with the PC group. (× 400 magnification, Scale bar = 50µm).
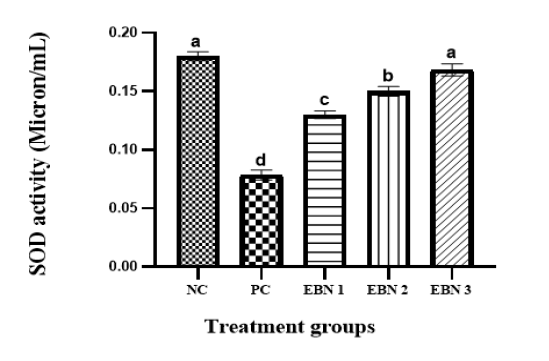
Figure 6: Plasma levels of SOD activity in NC, PC, and EBN treated groups. Values are mean ± SEM. Note: the increase in SOD activity in a dose-dependent manner with EBN supplement with all doses of EBN treatment showing a significant improvement compared to PC. P = 0.05.
Antioxidant activity
Figure 6 shows that plasma SOD activities were significantly (p<0.05) reduced in the PC group as compared to the NC group. However, in the EBN-treated rats, the SOD activities tended to increase, resulting in significantly higher (p<0.05) levels than the PC group. The rise in SOD activities in EBN-treated groups was dose-dependent, and enzymatic levels were elevated with an increase in EBN dose; it is worth noting that the levels in 120mg/kg (EBN 3) were restored to negative control levels as there was no significant difference (p>0.05) between these two groups. Cd has been reported to damage the anti-oxidative defense system (Järup, 2003). Moreover, this heavy metal interferes with some essential metals needed for anti-oxidant activities (Brzóska et al., 2016). Disruption of oxidative/antioxidative status in people exposed to this heavy metal was also documented, including tobacco smokers (Kiziler et al., 2007; Mezynska & Brzóska, 2018). SOD is the most effective and essential detoxifying enzyme in the cell. It is a key endogenous antioxidant enzyme that functions as a first-line defense system component against ROS. (Ighodaro & Akinloye, 2018). Our findings demonstrated that the SOD levels for rats treated with CdCl2 were significantly reduced in ovaries, as observed in earlier studies (Nna et al., 2017). It has been shown that EBN reduces the oxidation and peroxidation of membrane phospholipids (Quek et al., 2018). A significant rise in SOD action in the EBN treated groups was observed in the present study compared to the Cd-only treated group. These findings are consistent with the report of Albishtue et al. (2018), which showed that EBN could enhance AO enzymes and improve reproductive functions. It has well established that EBN is an excellent source of sialic acid and antioxidants (Quek et al., 2018). EBN can delay aging by increasing the activity of antioxidant enzymes and by reducing the content of lipid peroxidation products (Hu et al., 2016). These findings support our observation that increased activity of enzymatic antioxidants in supplementing EBN in Cd-intoxicated rats.
Conclusion
The current study concluded that the supplementation of EBN to the female rats could ameliorate Cd-induced reproductive toxicity and have promising activity towards reproductive performance. EBN showed antioxidant solid and tissue proliferating effects by increasing SOD levels and enhanced expressions of VEGF in ovarian tissues and promoting ovarian structures, respectively. Finally, it is recommended to explore the potential effects of EBN on several other reproductive parameters, as well as longer treatment periods with EBN, to evaluate the effectiveness of its long-term therapeutical applications in Cd-induced toxicity.
Acknowledgments
The authors would like to thank the Ministry of Higher Education Malaysia for funding this research under the Fundamental Research Grant Scheme with reference number - FRGS/1/2018/SKK08/UPM/02/6 and Vote no. 5540114. This research was funded by UNIVERSITI PUTRA MALYSIA, grant number GPIPS-9641900 and FRGS-5540114.
authors contribution
This study was intellectualized by AQ, NY, FFAJ, MMN, MWH and MA. The investigations were performed by AQ, MA and MAB under the supervision of NY, FFAJ, MMN, MWH and UK. Statistical analysis was carried out by AQ and NY. Original manuscript was written by AQ, reviews and editing were finalized by AQ, NY, FFAJ, MMN, MWH, MA and MSI. The final manuscript was read and approved by all authors.
References





