Journal of Animal Health and Production
Research Article
Effect of Long-Term Oral Exposure to Carmoisine or Sunset Yellow on Different Hematological Parameters and Hepatic Apoptotic Pathways in mice
Mohamed M. A. Hussein1, Ahmed Hamed Arisha2,3*, Eman M. Tayel1, Samar A. Abdo1
1Department of Biochemistry, Faculty of Veterinary Medicine, Zagazig University, 44519 Zagazig, Egypt; 2Department of Physiology, Faculty of Veterinary Medicine, Zagazig University, 44519 Zagazig, Egypt; 3Department of Animal Physiology and Biochemistry, Faculty of Veterinary Medicine, Badr University in Cairo (BUC), Badr City, Cairo, Egypt.
Abstract |Azo dyes such as carmoisine (Car) and sunset yellow (SY) are widely used synthetic pigments with many health issues reported. Yet, the different mechanisms associated with the detrimental effect of long-term oral administration with elevated levels of either Car or SY remain understudied. Several key endpoints were assessed including hematological, biochemical, and molecular biological parameters using a total of 100 adult Balb/c male mice. Five groups containing 20 mice each were orally administered comparable doses of either SY or Car for 12 weeks: Group (1) was a control group, group (2) were administered with the acceptable daily intake (ADI) limit of SY (4 mg/kg B.wt.), group (3) were administered with 10x of the ADI limit of SY, group (4) were administered with the ADI limit of Car (30 mg/kg B.wt) and group (5) were administered 10x of the ADI limit of Car. Exposure to higher doses of either SY or Car significantly (P < 0.05) altered the hematological parameters, decreased (P < 0.05) serum total protein (TP), albumin and globulin levels, increased (P < 0.05) liver enzymes, reduced (P < 0.05) hepatic antioxidant enzymes level and increased (P < 0.05) the lipid peroxidation marker malonaldehyde (MDA), upregulated (P < 0.05) the mRNA expression of the proapoptotic genes and down regulated (P < 0.05) the transcriptional level of the antiapoptotic gene Bcl2. Collectively, excessive oral administration of the two food coloring agents (Car and CY) for 12 weeks is hepatotoxic and shall be approached with caution.
Keywords | Carmoisine; Sunset yellow, Apoptosis; Caspase 3, Antioxidant enzymes
Received | July 21, 2021; Accepted | August 08, 2021; Published | August 25, 2021
*Correspondence | Ahmed Hamed Arisha, Department of Physiology, Faculty of Veterinary Medicine, Zagazig University, 44519 Zagazig, Egypt; Email: vetahmedhamed@zu.edu.eg
Citation | Hussein MMA, Arisha AH, Tayel EM, Abdo SA (2021). Effect of long-term oral exposure to carmoisine or sunset yellow on different hematological parameters and hepatic apoptotic pathways in mice. J. Anim. Health Prod. 9(s1): 80-86.
DOI | http://dx.doi.org/10.17582/journal.jahp/2021/9.s1.80.86
ISSN | 2308-2801
Copyright © 2021 Arisha et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Food additives are substance utilized to improve the final quality of food stuffs that are not typical ingredients of the food, although affecting their characteristics (Organization, 2013). Food additives have exceptionally essential role to supply the needs of any growing population for improving the taste, presentation, preservation or even their nutritive values. Countless chemical substances have been permitted to be utilized as food additives to enhance the
flavor, appearance as accepted by the Food and Drug Administration (FDA) in the United States and the European Food Safety Authority (EFSA) in European Union. Those chemicals comprising food pigments, preservatives, acid regulators, antioxidants, emulsifiers and stabilizers, flavor enhancers and others (Kramer et al., 2019). In Egypt, there is a severe surge in the use of synthetic colors particularly in the rural area owing to their higher stability and lower cost (Abd Elhalem et al., 2016; Ali et al., 2016).
Azo dyes, including carmoisine and sunset yellow, are the most implicated synthetic pigments causing health issues, because of their azo linkage (−N=N−) that is the most labile part of azo compounds that can be easily enzymatically broken during metabolism and liberating the free radicals with formation of stable aromatic amines, which are generally not degraded under anaerobic conditions causing oxidative stress (Pandey et al., 2007). Subsequently, various researchers paid attention for the toxicological and metabolic effect of those dyes either on animals or humans to evaluate their impacts on public health, and virtually all these studies proved that synthetic food dyes are harmful constituents for consumers, particularly during childhood (Diacu 2016; Elewa et al., 2019).
Carmoisine (E122) and sunset yellow (SY) (E110) are commonly utilized in ice cream, yoghurt, instant puddings, flavored fries, cake blends, candies, fermented dairy foodstuffs, pharmaceutical products and cosmetics (Tsai et al., 2015). Carmoisine (E122) is an azo dye (benzene-like structure) derived from coal-tar, which imply that it is a compound where two hydrocarbon groups are linked by two nitrogen atoms (Amin et al., 2010).
Reactive oxygen species (ROS) or reactive nitrogen species (RNS) contain group of molecules comprising molecular oxygen, nitrogen and their derivatives (Ryter et al., 2007). Excessive production of ROS, after surpassing the endogenous antioxidant defense mechanisms leading to oxidation of several biomolecules like proteins, DNA, lipids and carbohydrates (Arisha et al., 2019). Oxidative stress (OS) is the discrepancy among the free radicals load and the scavenging power of the antioxidant defense mechanism (Abdel-Daim et al., 2020). Excess amount of ROS could damage DNA, proteins, lipids, membranes and organelles such as mitochondria (Halliwell, 2011). Lipid peroxidation comprises a main result of cellular oxidative stress. It implies the addition of oxygen to unsaturated fatty acids resulting in the production of organic hydroperoxides. Increasing ROS levels elevates cysteine-aspartic acid protease-3 (caspase-3) level promoting an apoptotic state (Zhao et al., 2001; Zou et al., 2013).
Apoptosis is a vital process that eliminate unneeded cells during development or neutralizes potentially harmful cells via DNA damage (Fulda et al., 2010; Pallepati and Averill-Bates 2012). Caspase-3 cleavage is the crucial downstream apoptotic event. It is activated in the apoptotic cell both by the extrinsic and intrinsic pathways (Boatright and Salvesen, 2003; Jin and El-Deiry 2005). Such pathways involve the activity of several apoptotic modulators including the members of B-cell-lymphoma protein- 2 (Bcl-2) family, essential for mitochondrial integrity, whereby the balance of proapoptotic (Bax) and antiapoptotic (Bcl2) proteins regulates the cellular response to any apoptotic stimuli (McJilton et al., 2003). The main objective of this study was to explore the hematologic and hepatotoxic effects/ toxicity associated molecular mechanisms following long term oral administration of carmoisine or sunset yellow at two different doses in male mice.
Materials and methods
Animals
A total of 100 adult Balb/c male mice (average 20±2 g in weight) obtained from the Veterinary Animal House facility of Zagazig University. They were left for 15 days for acclimatization before any experimentation. All mice were maintained under the standard conditions as indicated by the guidelines of ZU-IACUC committee of Zagazig University, Egypt (ZU-IACUC/4/F/119/2020).
Experimental design
The experimental mice were randomly distributed into five groups (20 mice per group). SY was purchased from El-Gomhouria Co. (Cairo, Egypt) and Car was purchased from Lobachemie Co. (Mumbai, India). Group 1 (Control) was orally administered with distilled water (1ml/kg B.wt.). Groups 2 (SY LD) included mice that were orally administered with SY dissolved in distilled water (4 mg/kg B.wt.) as a low dose (LD) similar to the acceptable daily intake (ADI) limit (Reza et al., 2019). Groups 3 (SY HD) included mice that were orally administered with SY dissolved in distilled water (40 mg/kg B.wt.) equivalent to 10x of the ADI limit (high dose, HD). Groups 4 (Car LD) included mice that were orally administered with Car dissolved in distilled water (30 mg/kg B.wt.) as a low dose (LD) similar to the ADI limit (Aboelwafa and Ismail, 2017). Groups 5 (CAR HD) included mice that were orally administered with Car dissolved in distilled water (300 mg/kg B.wt.) equivalent to 10x of the ADI limit. The experiment lasted for 12 weeks.
Sample collection procedures
After 3 months, mice in each group were used for blood collection via the orbital venous plexus. The collected blood samples (with/ without anticoagulant) were either used for serum separation were performed via centrifugation at 3000 r.p.m for 20 minutes or sent as a whole blood for complete blood count (CBC) analysis. The serum (i.e. supernatant) were collected and stored at till analysis of biochemical parameters. Immediately after scarifying, liver were excised, weighted from all groups. Every sample was divided into two parts: One of them was wrapped in aluminum foil and put immediately in liquid nitrogen container to make snap-freezing of tissue for real time-PCR analysis. The other part was stored at -20oC for biochemical analysis of antioxidant enzymes/ lipid peroxidation marker.
Table 1: Specific real time PCR primers for examined genes.
| Gene |
Forward primer (5′–3′) |
Reverse primer (5′–3′) |
Accession No | Product size |
| Bax | CGAATTGGCGATGAACTGGA | CAAACATGTCAGCTGCCACAC | NM_017059.2 | 109 |
|
Bcl-2 |
GACTGAGTACCTGAACCGGCATC | CTGAGCAGCGTCTTCAGAGACA | NM_016993.1 | 135 |
| Fas | GAGCGTTCGTGAAACCGACA | AGGTTGGTGCACCTCCACTTG | NM_139194.2 | 128 |
| FasL | CACCAACCACAGCCTTAGAGTATCA | CACTCCAGAGATCAAAGCAGTTCC | NM_012908.1 | 172 |
| Caspase-3 | GAGACAGACAGTGGAACTGACGATG | GGCGCAAAGTGACTGGATGA | NM_012922.2 | 147 |
| GAPDH | GGCACAGTCAAGGCTGAGAATG | ATGGTGGTGAAGACGCCAGTA | NM_017008.4 |
143 |
Table 2: Effect of orally administered sunset yellow or carmoisine on different hematological parameters.
| Control | SY LD | SY HD | Car LD | Car HD | |
|
RBCs (×106/µl) |
7.03a ± 0.27 |
6.88a ± 0.36 |
4.41c ± 0.16 |
6.43a ± 0.25 |
5.67b ± 0.13 |
| Hb (gm%) |
16.5a ± 0.87 |
17.33a ± 0.81 |
10.79b ± 0.49 |
16.75a ± 0.43 |
11.38b ± 0.36 |
| PCV (%) |
38a ± 0.29 |
38.11a ± 0.084 |
24.63b ± 1.54 |
38.39a ± 0.29 |
29.68b ± 1.05 |
| MCV (fL) | 54.29 ± 2.54 | 55.70 ± 3.03 | 56.32 ± 5.61 | 59.88 ± 1.85 |
52.30 ± 0.95 |
| MCH (pg) | 23.66 ± 2.16 | 25.20 ± 0.13 | 24.48 ± 0.20 | 26.09 ± 0.32 |
20.10 ± 0.98 |
| MCHC (%) | 43.39 ± 1.95 | 45.48 ± 2.23 | 44.42 ± 4.80 | 43.62 ± 0.80 |
38.52 ± 2.59 |
|
PLT (× 103/μl) |
791.5b ± 19.57 |
857.5b ± 24.99 |
1079.5a ± 43.01 |
862.5b ± 24.33 |
986.5a ± 15.87 |
|
TLC (×103/µl) |
9.75b ± 0.72 |
9.0b ± 1.44 |
13.25a ± 0.43 |
9.5b ± 0.58 |
12.98a ± 0.30 |
| Basophils (%) |
0.22a ± 0.02 |
0.18a ± 0.04 |
0.12b ± 0.02 |
0.22a ± 0.02 |
0.17ab ± 0.01 |
| Eosinophils (%) | 0.38 ± 0.032 | 0.34 ± 0.072 | 0.26 ± 0.046 | 0.30 ± 0.03 |
0.27 ± 0.01 |
| Lymphocytes (%) |
83.25b ± 0.58 |
86.09b ± 2.07 |
89.93a ± 0.25 |
85.83b ± 0.19 |
86.23ab ± 0.99 |
| Monocytes (%) |
2.68b ± 0.10 |
3.25ab ± 0.72 |
3.75a ± 0.14 |
2.85b ± 0.09 |
3.55a ± 0.17 |
Data are expressed as means ± SEM. n=10. Means bearing different superscripts in a row were significantly different at P < 0.05. RBC: red blood cells, Hb: hemoglobin, PCV; packed cell volume, MCV: mean corpuscular volume, MCH: mean corpuscular hemoglobin, MCHC: mean cell hemoglobin concentration, TLC; total leucocytic count, PLT: platelets.
Biochemical measurements
Serum levels of total protein (TP), albumin or globulins were analyzed using kits (Biodiagnostic Company, Dokki, Giza, Egypt) following manufacturer’s guidelines. The serum levels of Alanine transaminase (ALT), Aspartate aminotransferase (AST), Alkaline phosphatase (ALP) were analyzed as previously reported (Arisha et al., 2019). Hepatic levels of superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), glutathione (GSH) and malondialdehyde (MDA) were performed as previously reported (Arisha and Moustafa, 2019).
Real time RT-PCR analysis
Total RNA was extracted using Trizol reagent (Invitrogen; Thermo Fisher Scientific, Inc.). For mRNA, a total of 1 µg of that resulting RNA was reverse-transcribed utilizing the HiSenScript™ RH (-) cDNA Synthesis Kit (iNtRON Biotechnology Co., South Korea) following manufacturer instructions. All implemented primers were imported from Sangon Biotech (Beijing, China) (Table 1) using Primer 3 for design. The real-time PCR was performed using Maxima SYBR Green/Rox qPCR Master Mix (2X) (ThermoFisher Scientific, Waltham, MA, USA) in a Mx3005P Real-Time PCR System (Agilent Stratagene, USA). Data were reported as a relative expression of each gene normalized to the housekeeping gene (Gapdh) was reported as fold change by a 2−ΔΔCT as a percentage from the control (Livak and Schmittgen, 2001).
Statistical analysis
Statistical analysis was applied using SPSS (IBM SPSS Statistics for Windows) version 24 and figures were generated with GraphPad prism 8 (San Diego, CA, United States). Different data shown as mean ± standard error of mean (SEM). Statistical comparisons between groups were utilized using one-way analysis of variance (ANOVA) followed by post hoc Tukey test. A P-value < 0.05 implies the presence of statistical significant.
Results
Effect of orally administered sunset yellow or carmoisine on different hematological parameters
A significant (P < 0.05) reduction in RBCs, Hb and PCV values were noticed following chronic oral administration of high dose, but not low dose, of either SY (40 mg/Kg B.wt.) or Car (300 mg/Kg B.wt.) as shown in table 2. No significant (P > 0.05) alteration in the different blood cell indices were noticed following administration of either SY or Car at both doses as shown in Table 2. A significant (P < 0.05) elevation in platelet count, total leucocytic count and monocytes (%) were noticed following oral administration of high dose, but not low dose, of either SY (40 mg/Kg B.wt) or Car (300 mg/Kg B.wt) for three months as shown in (Table 2). A significant (P < 0.05) decrease in basophils (%) as well as a significant (P < 0.05) elevation in lympocytes (%) was noticed following oral exposure of SY (40mg/Kg B.wt.) for 12 weeks.
Effect of orally administered sunset yellow or carmoisine on total protein, albumin and globulin levels
A significant (P < 0.05) reduction in TP, albumin and globulin levels were noticed following chronic oral administration of high dose, but not low dose, of either SY (40mg/Kg B.wt.) or Car (300mg/Kg B.wt.) (Fig 1A-C). No significant alteration (P > 0.05) in A/G ratio was noticed following administration of either SY or Car at both doses (Fig 1D).
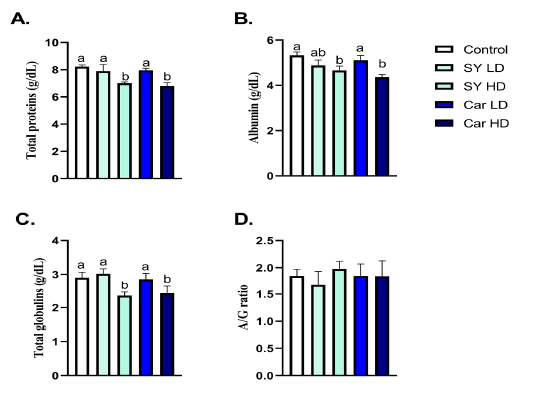
Figure 1: Effect of orally administered sunset yellow or carmoisine on total protein, albumin and globulin levels. A. Total protein (g/dL), B. Albumin (g/dL), C. Globulin (g/dL) and D. Albumin/Globulin ratio. Values are mean ± SEM of 10 animals per experimental group. Means with distinct superscripts were statistically significantly at P < 0.05.
Effect of orally administered sunset yellow or carmoisine on liver enzymes
A significant (P < 0.05) increase in ALT, AST and ALP were noticed following chronic oral administration of high dose of either SY (40 mg/Kg B.wt.) or Car (300 mg/Kg B.wt.) (Fig 2A-C). A significant (P < 0.05) elevation in ALT was noticed following chronic oral administration of low dose of either SY (4 mg/Kg B.wt.) or Car (30 mg/Kg B.wt.) (Fig 2A). A significant (P < 0.05) raise in ALP was noticed following chronic oral administration of low dose of Car (30mg/Kg B.wt.), but not SY (4mg/Kg B.wt.), (Fig 2B). A significant (P < 0.05) elevation in AST was noticed following chronic oral administration of low dose of SY (4mg/Kg B.wt.), but not Car (30mg/Kg B.wt.), (Fig 2C).
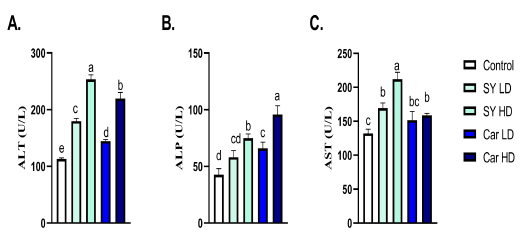
Figure 2: Effect of orally administered sunset yellow or carmoisine on liver enzymes. A. ALT (U/L), B. ALP (U/L) and C. AST (U/L). Values are mean ± SEM of 10 animals per experimental group. Means with distinct superscripts were statistically significantly at P < 0.05.
Effect of orally administered sunset yellow or carmoisine on the hepatic antioxidant enzymes/ lipid peroxidation levels
A significant (P < 0.05) reduction of the hepatic SOD, CAT, GSH and GPx levels as well as elevation in MDA were noticed following chronic oral administration of high dose of either SY (40mg/Kg B.wt.) or Car (300mg/Kg B.wt.) were shown (Fig 3A-E). A significant (P < 0.05) decrease in hepatic CAT and GSH levels, but not SOD and GPx, as well as elevation in MDA were noticed following chronic oral administration of low dose of Car (30mg/Kg B.wt.) (Fig 3A-E). No significant (P > 0.05) alteration in hepatic SOD, CAT, GSH, GPx and MDA levels were noticed following chronic oral exposure to the lower SY dose (4 mg/Kg B.wt.) (Fig 3A-E).
Effect of orally administered sunset yellow or carmoisine on the hepatic transcriptional levels of apoptotic genes
Chronic oral administration of high dose of either SY (40mg/Kg B.wt.) or Car (300mg/Kg B.wt.) showed significant (P < 0.05) up-regulation in hepatic mRNA expression of apoptotic markers (Bax, Fas, FasL and Caspase-3) as well as downregulation in hepatic mRNA expression of anti-apoptotic gene Bcl2 when compared with control group (Fig 4A-E). A significant (P < 0.05) up-regulation in hepatic mRNA expression of Bax was noticed following chronic oral administration of low dose of SY (4mg/Kg B.wt.) (Fig 4A). A significant (P < 0.05) up-regulation in hepatic mRNA expression of Bax and FasL were noticed following chronic oral administration of low dose of Car (30mg/Kg B.wt.) (Fig 4A and C).
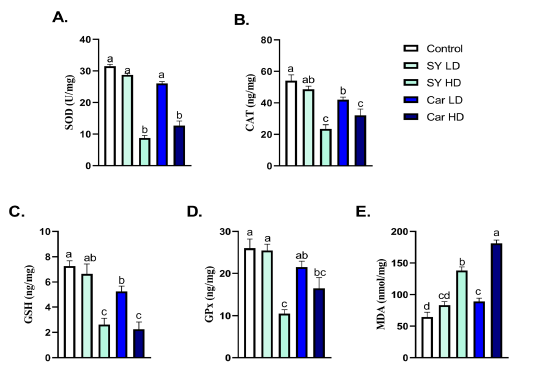
Figure 3: Effect of orally administered sunset yellow or carmoisine on hepatic antioxidant enzymes and lipid peroxidation. A. SOD (U/mg), B. CAT (ng/mg), C. GSH (ng/mg), D. GPx (ng/mg) and E. MDA (nmol/mg). Values are mean ± SEM of 10 animals per experimental group. Means with distinct superscripts were statistically significantly at P < 0.05.
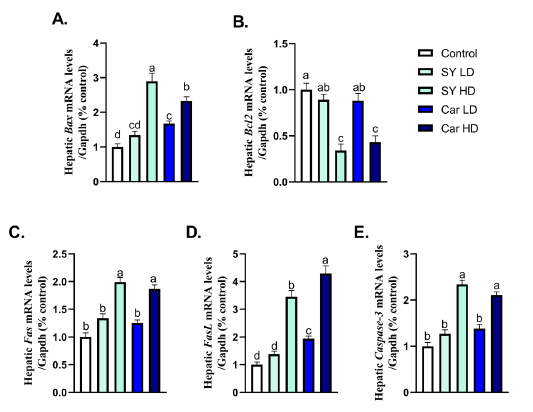
Figure 4: Effect of orally administered sunset yellow or carmoisine on the hepatic transcriptional levels of apoptotic genes. A. Bax, B. Bcl2, C. Fas, D. FasL and E. Caspase-3. Values presented are mean ± SEM. Means with distinct superscripts were statistically significantly at P < 0.05.
Discussion
Colors play a vital role in food products evaluation by consumers, though higher stability during storage and lower cost of such synthetic food colorants offer various side effects were reported as hyperactivity, allergic conditions or even cancers. Although consumer preferences has shifted from using synthetic dyes, no alternative dye of natural origin has been fully valid (Sen et al., 2019; Sigurdson et al., 2017). Countless chemical substances have been permitted to be utilized as food additives in order to enhance flavor, appearance and even products consistency. Those chemicals comprising preservatives, food pigments, antioxidants and acid regulators as well as flavor enhancers emulsifiers and stabilizers (Kramer et al., 2019). The acceptable daily intake for sunset yellow as suggested by EFSA is 4 mg/kg B.wt./day (Additives and Food 2014).
Similar to our results, previous reports showed that following treatment with either SY/Car or their degradation products significantly changed the different hematological parameters including RBCs count, Hb, PCV, MCV, MCH, MCHC, blood PLTs, and WBCs (Elbanna et al., 2017). Furthermore, ALT and AST were significantly increased in rats treated with such azo dyes in comparison with either recovery and control groups (Elbanna et al., 2017).
The adverse effects of the SY as a synthetic food coloring agent were also previously investigated by different researchers such as (Hashem et al., 2011) showed that a dosage of 47 mg/kg BW of «amaranth and SY» in rats could alter the hepatic function and had better to be avoided throughout the pregnancy. Likewise, (Sayed et al., 2012) proved the mutagenic action of SY dye at dosage of 0.325 mg/kg BW/day for 1, 2 or 3 weeks and the protective role of Se and A, C and E vitamins in mice with significant chromosomal aberration values in the hepatic tissues. Ismail and Sakr (2016) reported drastic ultra-structural effects in the hepatocytes, chromosomal aberrations and the frequency of Mitotic Index (MI) of the cells of bone marrow. Moreover, SY (E110) affect the reproductive criteria in mouse and not safe as natural colorant CU (E100) due to higher level of ROS produced after their intake which accompanied by imbalance in oxidative status (Ismail, 2016). Carmoisine may produce the deleterious effects through induction of lipid peroxidation and suppression of antioxidant enzymes activity (Montaser et al., 2018), Moreover, it induces a genotoxicity through induction of DNA damage (Khayyat et al., 2017) likewise (Montaser et al., 2018) reported that carmoisine consumed at different levels higher than the acceptable daily-authorized level caused harmful effects on fertility. (Valentovic et al., 2002) postulated that aromatic amines incriminated in the carcinogenic and endotoxic effects of azodyes and significantly reduced GSH levels. (Amin et al., 2010) demonstrated that, in young male rats, the high doses of tartrazine (as example of azo dye; 500 mg/kg B.wt.) significantly decreased hepatic GSH levels. Incubation of hepatocytes with aromatic amines resulted in a reduction in the mitochondrial membrane potential (Siraki et al., 2002). Furthermore, depletion of hepatic GSH was shown with all arylamines tested. Furthermore, exposure to low or high doses of Car and tartrazine did not significantly change hepatic GSH content and MDA levels (Cemek et al., 2014). Food additives affect the apoptotic marker as suggested wher Ascorbyl palmitate accelerates apoptosis by up-regulating the levels of caspase-3/ 9 and down-regulating Bcl-2 (Qu et al., 2019; Sohrabi et al., 2018).
Conclusions
Current investigation exhibited that long-term oral administration of food coloring agents (Car and CY) is hepatotoxic and have negative effects on immunity. Although coloring agents including Car and CY are commercially used/ added to food for different purposes, their use should be following strict approach. Such food colors should only be used at a safe level as indicated by the ADI limits.
Conflict of interest
No conflicts of interest to declare.
Authors contribution
All authors performed the experiments, analyzed and revised the data, interpreted the results and drafted the manuscript.
References





