Journal of Animal Health and Production
Research Article
Growth Performance, Proximate Composition and Immune Competence of Naked Neck, Rhode Island Red and their F1 Crossbred Chickens in a Tropical Climate
Mohammad Alam1*, Naila Chand1, Sarzamin Khan1, Syed Muhammad Suhail2
1Department of Poultry Science, Faculty of Animal Husbandry and Veterinary Sciences, The University of Agriculture, Peshawar, 25120, Khyber Pakhtunkhwa, Pakistan; 2Department of Livestock, Animal Breeding and Genetics, Faculty of Animal Husbandry and Veterinary Sciences, The University of Agriculture, Peshawar, 25120, Khyber Pakhtunkhwa, Pakistan.
Abstract | A study was conducted on the growth performance, carcass characteristics and immune competence of Naked Neck (NN), exotic Rhode Island Red (RIR) and their crossbred NNRIR chickens in the tropical summer months of May to September at a natural ambient temperature of 25.4°C to 38.2°C. Naked Neck gene (Na) was introduced into exotic RIR chickens by crossing cockerels of RIR with homozygous NN pullets to produce their crossbred NNRIR chickens. Growth performance significantly (P<0.05) varied among the different genetic groups. Crossbred NNRIR gained 6.97% more body weight and consumed 4.95% less feed than indigenous NN birds. Total feed consumption ratio (day1-20 weeks of age) of crossbred NNRIR was 9.50% lower (better) than indigenous NN birds. Dressing percentage was significantly (P<0.05) higher and same for exotic RIR (63.5%) and NNRIR (62.9%) while lowest for indigenous NN (60.7%). Significantly higher (P<0.05) thigh and drumstick meat dry matter (DM) was recorded for exotic RIR (30.1% and 30.5%, respectively). Significantly (P<0.05) higher crude protein (CP) content was recorded in thigh meat of crossbred NNRIR (67.1%) and drumstick meat of exotic RIR (66.1%). Immune competence traits such as cell mediated immune response (CMI), humoral immunity and phagocytic activity, were significantly higher (P<0.05) for crossbred NNRIR as compared to RIR chickens. The results indicated that crossbred NNRIR performed better than indigenous NN in terms of growth performance and were relatively more heat tolerant than exotic RIR chickens. It was concluded that cross breeding has potential for improving economically important traits of birds in tropical regions without compromising their adaptability to environmental stressors.
Keywords | Naked neck, Rhode Island Red, Crossbreeding, Meat composition, Immunity, Stress
Editor | Asghar Ali Kamboh, Sindh Agriculture University, Tandojam, Pakistan.
Received | March 08, 2021; Accepted | June 30, 2021; Published | August 25, 2021
*Correspondence | Mohammad Alam, Department of Poultry Science, Faculty of Animal Husbandry and Veterinary Sciences, The University of Agriculture, Peshawar, 25120, Khyber Pakhtunkhwa, Pakistan; Email: m.alam@aup.edu.pk
Citation | Alam M, Chand N, Khan S, Suhail SM (2021). Growth performance, proximate composition and immune competence of naked neck, Rhode Island Red and their F1 crossbred chickens in a tropical climate. J. Anim. Health Prod. 9(3): 303-311.
DOI | http://dx.doi.org/10.17582/journal.jahp/2021/9.3.303.311
ISSN | 2308-2801
Copyright © 2021 Alam et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Backyard poultry production is an important segment of poultry production in the tropics. Backyard poultry keeping is an important economic activity carried out by almost 80 percent families in rural areas of Pakistan (FAO, 2003). It contributes about 8.6 and 23.5 percent, respectively, of the total poultry meat and eggs produced in the country (GoP, 2018). In most of the developing countries, indigenous poultry genotypes constitute about 80 to 99 percent of the total poultry populations maintained in villages (Sonaiya and Swan, 2004). In Pakistan, backyard poultry is mostly confined to rural areas and include Aseel, Naked Neck (NN), Fayoumi, Desi (non-descript) and their crosses. Majority of these birds are reared mostly by women and children of poor families (Sadef et al., 2015). Indigenous chickens provide substantial amount of poultry meat and eggs to rural populations (Ibrahim et al., 2019) despite being poor performers than improved commercial strains (Dessie et al., 2003; Islam, 2006; Sadef et al., 2015). Indigenous breeds possess great potential for both sustainable productions and future breeding programs (Sahota and Bhatti, 2003; Besbes, 2009). Among the indigenous chicken breeds, the NN strain is well known for better performance, significantly higher body weight and egg production associated with substantial weight and mass output (Islam, 2006; Galal et al., 2007; Fathi et al., 2008; Sharifi et al., 2010). Several researchers have investigated how the Na gene is linked with high egg and meat production. The improved heat dissipation effect of Na gene (Patra et al., 2002; Rajkumar et al., 2010b) significantly increases appetite. i.e. increased feed intake (Cahaner et al., 1993), followed by higher weight gain (Fathi et al., 2008; Reddy et al., 2008; Rajkumar et al., 2010a), egg weight (El-Safty, 2006; Mahrous and El-Dlebshany, 2011) and livability (Islam, 2006) under high temperatures. The most likely use of the Na gene is at high mean ambient temperatures, i.e. 25 ℃ and above, where it may lead to higher growth rate, slaughter yield, meat yield, and resistance to acute heat stress (Merat, 1990). Incorporating Na gene in a breed increases the egg weight, egg number, egg mass and shell strength (El-Safty, 2006). The Na gene reduces total body feathering by up to 20-40% (Singh et al., 2001; Fathi et al., 2008) and is involved in heat tolerance (Isidahomen et al., 2012).
Exotic chickens exhibit high productivity in terms of meat and egg production as compared to indigenous chicken, however, these are not adapted to stressful environmental conditions such as high temperatures, diseases and poor nutrition (Kgwatalala and Segokgo, 2013). Crossbreeding NN chickens with fast-growing commercial RIR (Rhode Island Red) birds could result in better performing and disease resistant birds (Adebambo et al., 2010). The ideal crossbred chicken would have better productivity and adaptability to local harsh environmental conditions (Adebambo et al., 2011). This study was therefore, conducted to evaluate the performance and stress status of NN and its crossbreds with RIR in tropical summer conditions for future identification of climate smart chicken in the global climate change scenario.
MATERIALS AND METHODS
This experiment was designed to determine the growth performance, carcass characteristics, immune response, and stress parameters of Naked Neck (NN), Rhode Island Red (RIR) and F1 crossbred of NN pullet with RIR cockerel (NNRIR).
Location
The experiment was conducted at The University of Agriculture, Peshawar, Khyber Pakhtunkhwa, Pakistan. Peshawar is located between 34° 1’ 33.3012’’ N and 71° 33’ 36.4860’’ E at a height of 331 m from the sea level. The temperature ranges from 4 °C in winter to 40°C in summer seasons.
Experimental birds
Naked neck gene was introduced into exotic RIR chicken by crossing with a homozygous Naked Neck (NaNa) population for improving their production under tropical conditions. The base population of NN line was provided by National Agriculture Research Council where it is maintained. A total 360 birds (120 each of NN, RIR and NNRIR) were hatched out from the above population in a single hatch and randomly distributed at the rate of 5 birds per battery brooder pen (60×75 cm) placed in an open sided house.
Housing and management
Chicks were kept under similar management and nutritional conditions with a brooding temperature of 34±1°C to 26±1°C from first to third week of rearing, after that, the birds were kept at a natural ambient temperature (25.4 to 38.2°C) of summer for twenty weeks of age (May to September). The experimental birds were provided free access to clean and fresh drinking water through drinkers and fed balanced commercial ration, formulated according to National Research Council (1994) standards (Table 1) and recommendations made by Leeson and Summers (2005) (Table 2). The chicks were vaccinated against Marek’s disease (Day one), Newcastle disease (7th and 35th day), Infectious Bronchitis (9th day) and infectious bursal disease (14th and 24th day). The birds were reared at a natural ambient temperature (25.4 to 38.2°C) of summer. The experiment was approved by the Faculty Animal Ethics Committee.
Table 1: Ingredient composition of starter and grower ration.
| Ingredients (g/ kg) | Starter ration | Grower ration |
| Yellow Maize | 450 | 470 |
| Soybean meal | 130 | 115 |
| Rice | 40 | 40 |
| Canola Meal | 62 | 60 |
|
Rice polish |
125 | 130 |
| Wheat bran | 90 | 100 |
| Sunflower meal | 80 | 60 |
| Dicalcium phosphate | 6.0 | 8.0 |
| Salt | 2.0 | 2.0 |
| Limestone | 10.0 | 10.0 |
| Bone meal | 5.0 | 5.0 |
| Total | 1000 | 1000 |
Table 2: Chemical composition (nutritive values) of starter and grower ration.
| Composition (g/ kg) | Starter ration | Grower ration |
| Crude protein | 19.5 | 17.0 |
| Crude fiber | 5.42 | 5.48 |
| Ether extract | 4.28 | 4.21 |
| Calcium | 0.59 | 0.84 |
| Phosphorus | 0.37 | 0.42 |
| Lysine | 1.23 | 1.21 |
| Methionine and Cysteine | 0.94 | 0.88 |
| Threonine | 0.87 | 0.9 |
| Metabolizable Energy (Kcal/ kg) | 2780.4 | 2993.3 |
Growth performance
Average weight of each group was computed on day first of the experiment by weighing each replicate group. Data was recorded for body weight gain at two weeks’ interval for twenty weeks of age. Initial weight was subtracted from final weight to compute weight gain in the respective week. Feed intake was recorded on daily basis for each replicate group throughout the experiment. For this purpose, data on daily feed offered and refused was recorded. Daily feed intake was used to workout weekly and total feed intake. Feed conversion ratio (FCR) was calculated fortnightly for weight gain during the experimental period.
Growth performance data was grouped into three phases, i.e., brooding phase (day1-8 weeks), growing (9-20 weeks) phase and pre-laying (day 1-20 weeks) phase.
Carcass characteristics
At 20 weeks of age, 36 birds were randomly picked from the experimental groups and euthanized for carcass traits and meat composition as percent of dressed weight. Proximate analysis was performed according to Horwitz (2015) for determination of breast, thigh and drumstick meat composition on dry matter basis.
Cell mediated immune response against phytohemagglutinin (PHA-P)
To evaluate the in vivo T-cell mediated immune response, twelve birds from each genetic group were randomly collected at the age of three months and injected with a mitogen phytohemagglutinin (PHA-P) solution in the wattle using the methodology of Grasman (2010). Briefly, A 100 μg of PHA-P in 0.1 ml sterile saline was injected in the left wattle and 0.1 ml of normal saline was injected in right wattle as control. Measurement of the thickness of wattle swelling was recorded before (T1) and after 24 and 48 hours (T2) of injection using a pressure sensitive micrometer. Wattle swelling and wattle index was calculated by the following formulae:
Wattle swelling (WS) = wattle thickness at T2 - wattle thickness at T1
Wattle index (WI) = WS of the left wattle - WS of the right (control) wattle
Mononuclear phagocytic system (Carbon clearance) function assay
Phagocytic immune response of experimental birds was evaluated by the carbon clearance assay (CCA) following the procedure described by Fathi et al. (2003). At the age of three months, a total of twelve birds per group were injected with supernatant of a commercial Black India ink centrifuged at 5000 rpm at 1 ml/kg in the right-wing vein. Blood from the left-wing vein was collected before the injection and at three and 15 minutes after the injection and transferred into 4 ml aqueous sodium citrate (2%) solution. After centrifugation of the samples at 5000 rpm for four minutes, the supernatant was observed through spectrophotometry at 640 nm for optical density (OD) measurement.
Humoral immune response against Sheep red blood cells (SRBCs)
Humoral immune response against SRBCs was evaluated according to the procedure described by Grasman (2010). Briefly, at the age of three months, one ml of 3% v/v SRBC/PBS was injected intramuscularly to a total of twelve randomly picked birds from each genetic group. Blood samples were collected from the selected birds before and seven, 14 and 21 days after SRBC injection. The samples were examined for hemagglutination and the reciprocal of highest dilution showing complete agglutination was expressed as antibody titre (log2).
Statistical analysis
Overall data for the three genetic groups was computed in Excel program of Microsoft Office 2016. For analysis of variance (ANOVA) of growth performance, a completely randomized design involving three genetic groups was used. Treatment means for each parameter were compared using Tukey multiple comparison test. IBM SPSS Statistics v. 25 was used for statistical analysis. The following statistical model was used for analysis data.
Yij = µ+ Gi + eij
Where,
Yij = yield subjected to individual observation; µ= overall true mean effect; Gi= effect of ith genetic group (NN, RIR, NNRIR); eij = Experimental error, called random error or residual.
RESULTS AND DISCUSSION
Growth performance
Growth performance in terms of body weight gain, feed intake and FCR of NN, RIR and their crossbred (NNRIR) is presented in Table 3. Significant differences (P<0.05) were recorded in the hatch weight of the three genetic groups.
Weight gain was significantly (P<0.05) higher for exotic RIR during brooding (0.48 kg), growing (0.90 kg) and pre-laying (1.38 kg) period, followed by crossbred NNRIR (0.44 kg, 0.80 kg, and 1.24 kg, respectively), while lowest weight gain was recorded for the indigenous NN (0.41 kg, 0.74 kg and 1.16 kg, respectively) (Table 3). Total weight gain of RIR birds was 16.80% higher than the indigenous NN, while that of NNRIR was 6.97% higher than the indigenous NN birds.
Feed intake of NN, RIR and their crossbred (NNRIR) are presented in Table 3. Feed intake was significantly higher (P<0.05) for indigenous NN (1.54 kg) while lowest and same for the exotic RIR (1.37 kg) and crossbred NNRIR (1.37 kg) during brooding period (Table 3). During growing (9-20 weeks) period, feed intake was significantly higher (P<0.05) for indigenous NN (5.43 kg) and lowest for the exotic RIR (5.03 kg) while that of NNRIR (5.25 kg) was not significantly different (P>0.05) from NN and RIR birds. Similar tendency was recorded during pre-laying (day 1-20 weeks) period. During pre-laying (day 1 to 20 weeks) period, RIR pullets consumed 8.15% while NNRIR pullets consumed 4.95% less feed than indigenous NN birds.
Feed conversion ratio (FCR) of NN, RIR and their crossbred (NNRIR) during the experimental period is shown in Table 3. The FCR of all three genetic groups increased with increasing age due to consumption of more feed with respect to age of the birds. Feed conversion ratio was significantly different among the genetic groups.
During the brooding period (day1-8 weeks of age), the exotic RIR had significantly lower (better) (P<0.05) FCR (2.83), followed by crossbred NNRIR (3.11) while higher (poor) FCR was recorded for the indigenous NN (3.73). During growing period (9-20 weeks of age), significantly better FCR was recorded for the exotic RIR chicken (5.56), followed by the crossbred NNRIR (6.55), while poor FCR was recorded for the indigenous NN (7.31). Similar trend was recorded for total FCR. Total FCR (0-20 weeks) of crossbred NNRIR was 9.50% lower (better) than indigenous NN birds.
Carcass characteristics
Carcass characteristics of different genetic groups varied significantly (P<0.05) as shown in Table 4. Dressing percentage of indigenous NN (60.7%) was significantly lower (P<0.05) than that recorded for exotic RIR (63.5%) and crossbred NNRIR (62.9%).
Different meat portions differed significantly (P<0.05) in different genetic groups, with higher breast meat for the crossbred NNRIR (24.9%), thigh meat for indigenous NN (15.3%) while higher and same drumstick meat was recorded for indigenous NN (9.60%) and exotic RIR (10.1%).
Meat composition
Chemical composition of different meat portions of different genetic groups is presented in Table 5. Breast meat composition was not significantly different (P>0.05) however, significant differences (P<0.05) were recorded in chemical composition of thigh and drumstick meat of different genetic groups. Significantly higher thigh and drumstick meat dry matter (DM) were recorded for the exotic RIR (30.1% and 30.5%, respectively), while that of
Table 3: Growth performance of Naked Neck (NN), Rhode Island Red (RIR) and their crossbred (NNRIR) chicken.
| Growth Phase | NN | NNRIR | RIR | Pooled SEM | P-value | |
| Hatch weight (g) |
28.3b |
30.9a |
31.0a |
0.520 | <0.01 | |
| Total weight gain per bird (kg) | Brooding phase |
0.41c |
0.44b |
0.48a |
0.020 | <0.01 |
| Growing phase |
0.74c |
0.80b |
0.90a |
0.021 | <0.01 | |
| Pre-laying phase |
1.16c |
1.24b |
1.38a |
0.033 | <0.01 | |
| Feed intake per bird (kg) | Brooding phase |
1.54a |
1.36b |
1.37b |
0.035 | <0.01 |
| Growing phase |
5.43a |
5.25ab |
5.03b |
0.068 | 0.025 | |
| Pre-laying phase |
6.97a |
6.61ab |
6.40b |
0.094 | 0.013 | |
| FCR | Brooding phase |
3.73a |
3.11b |
2.83c |
0.131 | <0.01 |
| Growing phase |
7.31a |
6.55b |
5.56c |
0.342 | 0.032 | |
| Pre-laying phase |
6.03a |
5.33b |
4.61c |
0.233 | <0.01 |
a,b,c Means in the same row not sharing the same superscript are significantly different at p<0.05. NN, Naked Neck; RIR, Rhode Island Red; NNRIR, Crossbred of NN pullet and RIR cockerel, SEM, Standard error in mean.
indigenous NN (28.3% and 29.8%, respectively) and crossbred NNRIR (28.9% and 29.1%, respectively) were the lowest and same. Crossbred NNRIR had higher thigh and drumstick meat crude protein (CP) (67.1% and 61.4%, respectively) and less thigh and drumstick meat fat percentage (19.2% and 19.4%, respectively) as compared to indigenous NN. Thigh and drumstick meat CP of exotic RIR (66.2% and 66.1%, respectively) were insignificant to both NN and NNRIR while fat content of exotic RIR and crossbred NNRIR varied insignificantly across thigh and drumstick meat. Insignificant (P>0.05) differences were recorded for the ash content of all the three genotypes.
Table 4: Mean Carcass characteristics of Naked Neck (NN), Rhode Island Red (RIR) and their crossbred (NNRIR) chicken.
| Parameter | NN | NNRIR | RIR | Pooled SEM | P value |
| Live weight (kg) |
1.35c |
1.46b |
1.62a |
0.051 | <0.01 |
| Carcass weight (kg) |
0.78c |
0.91b |
1.03a |
0.042 | <0.01 |
| Dressed weight (%) |
60.7b |
62.9a |
63.5a |
0.44 | <0.01 |
| Breast weight (%) |
23.1b |
24.9a |
24.0b |
0.21 | <0.01 |
| Thigh weight (%) |
15.3a |
14.7b |
15.1b |
0.19 | <0.01 |
| Drumstick weight (%) |
9.6a |
9.2b |
10.1a |
0.14 | <0.01 |
a,b,c Means in the same row not sharing the same superscript are significantly different at P<0.05. NN, Naked Neck; RIR, Rhode Island Red; NNRIR, Crossbred of NN pullet and RIR cockerel, SEM, Standard error in mean.
Table 5: Meat composition of Naked Neck (NN), Rhode Island Red (RIR) and their crossbred (NNRIR) chicken
| Parameter | Composition (%) | NN | NNRIR | RIR | Pooled SEM | P value |
| Breast meat | DM | 27.1 | 27.0 | 27.6 | 0.16 | 0.315 |
| CP | 81.1 | 81.8 | 82.4 | 0.42 | 0.837 | |
| Fat | 6.85 | 7.07 | 6.87 | 0.121 | 0.755 | |
| Total Ash | 5.79 | 5.82 | 5.08 | 0.122 | 0.153 | |
| Thigh meat | DM |
28.3b |
28.9b |
30.1a |
0.30 | <0.01 |
| CP |
66.0b |
67.1a |
66.2ab |
0.20 | 0.046 | |
| Fat |
21.8a |
19.2b |
18.2b |
0.42 | <0.01 | |
| Total Ash | 7.13 | 6.87 | 7.06 | 0.124 | 0.690 | |
| Drumstick meat | DM |
29.8b |
29.1b |
30.5a |
0.65 | <0.01 |
| CP |
65.3b |
66.4a |
66.1ab |
0.97 | <0.01 | |
| Fat |
21.3a |
19.4b |
19.8c |
0.71 | <0.01 | |
| Total Ash | 5.81 | 5.55 | 5.87 | 0.141 | 0.634 |
a,b,c Means in the same row not sharing the same superscript are significantly different at P<0.05. NN, Naked Neck; RIR, Rhode Island Red; NNRIR, Crossbred of NN pullet and RIR cockerel, SEM, Standard error in mean; DM, Dry matter; CP, crude protein.
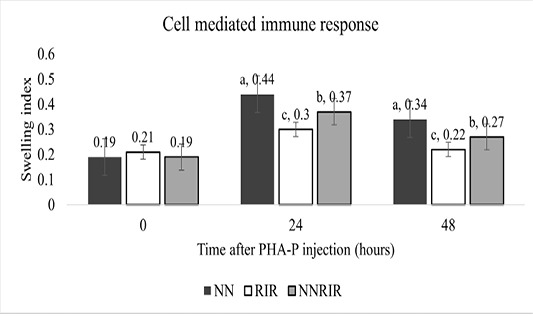
Immune competence
Cell mediated immune response of different genetic groups is shown in Figure 1. There was significant effect of genotype on the immune response of different genetic groups to injectable PHA-P. The immune response against PHA-P was significantly higher for the purebred Naked Neck followed by its crossbred NNRIR while the lowest response was recorded for the exotic RIR. Swelling response of crossbred NNRIR was 23.3% and 33.3% more than the exotic RIR chicken after 24 and 48 hours of PHA-P injection.
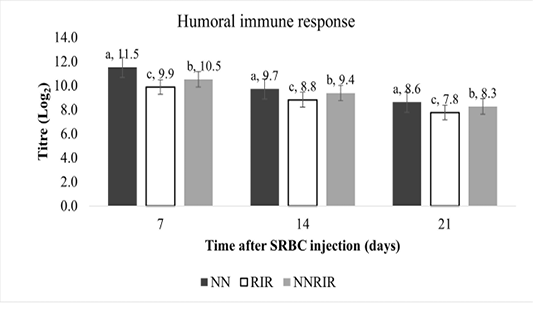
Humoral immune response of different genetic groups against sheep-red-blood cells (SRBC) is shown in Figure 2. Indigenous NN had significantly higher (P<0.05) total antibody titre at 7, 14 and 21 days post SRBC injection, followed by crossbred NNRIR while statistically lower antibody titre was recorded for exotic RIR chickens. Total antibody titre of crossbred NNRIR was 5.98 to 6.20% higher than the exotic RIR chickens.
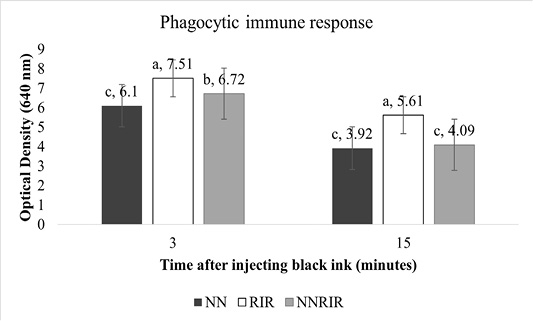
Phagocytic immune response of different genetic groups is shown in Figure 3. Significantly higher (P<0.05) response was shown by indigenous NN chickens while NNRIR crossbred performed better than the exotic RIR. Optical density of NNRIR crossbred was 10.5% and 27.1% lower than RIR chickens at 3 and 15 minutes post injection, showing better capability in clearing foreign antigens.
Findings of the present study revealed significant (P<0.05) effect of genotype on hatch weight among the genetic groups. These results are consistent with previous findings of Das et al. (2016), who reported higher day-old chick weight in RIR (34.8 g) as compared to local or crossbred chicken. Khawaja et al. (2012) recorded significantly higher day old weight for RIR (31.3 g) as compared to Fayoumi (20.9 g) and their crossbreds (25.2 to 30.0 g). Similarly, Malago and Baitilwake (2009) reported higher day old weight of RIR (30.1 g) as compared to local Tanzanian chicken breeds.
Growth performance
Body weight gain differed significantly (P<0.05) in different genetic groups during pre-laying period. The exotic RIR performed better as compared to other genotypes, while the crossbred NNRIR performed better than the NN genotype. The high body weight observed in NNRIR crossbred compared with NN chickens might be attributed to the genetic superiority of the RIR in body weight that is a highly heritable trait and known for its non-additive genetic response to crossbreeding (Alewi and Melesse, 2013). Findings of the present study are in line with the results of Das et al. (2014b), who reported higher body weight for RIR and its crosses as compared to local breeds. Similarly, Adebambo et al. (2010) reported that exotic breeds and its different genotypes performed better than indigenous breeds in terms of growth rate. The present results are comparable with those reported by (Khawaja et al., 2012), who reported highest bodyweight gain in exotic RIR and its crossbreds with Fayoumi, while lowest body weight gain was recorded in Fayoumi. Similarly, RIR × Fayoumi crossbred performed better than the purebred Fayoumi in terms of growth performances under intensive system (Azharul et al., 2005).
Feed conversion ratio of all three genetic groups increased with increasing age due to consumption of more feed with respect to age of the birds. Feed conversion ratio was significantly (P<0.01) lower (better) for the exotic RIR chickens, followed by the crossbred NNRIR, while poor FCR was recorded for the indigenous NN chickens. This improved FCR in crossbred NNRIR chickens might be attributed to the fact that crossbreeding results in heterosis which is mostly beneficial and is shown in the performance of the hybrids (Ekka et al., 2016). Das et al. (2014a) reported 8th week FCR of RIR strains as 4.02 and 5.21 for cockerels and pullets respectively which is higher than the results of the present study. Similarly, Mengesha (2012) reported average feed conversion ratio of some local chicken breeds as 7.0 and 4.2 at 8th and 12th weeks of age in intensive rearing system and 3.04 and 5.6 at 12th week of age in semi-intensive rearing system in the tropical countries of Africa. In contrast to our findings, Haque et al. (1999) reported better growth (1142.4 g) and feed conversion efficiency (5.10) for the crossbred NNRIR as compared to pure exotic breeds and other crosses. Khawaja et al. (2012) reported better FCR for exotic RIR and its crosses with Fayoumi, while poor FCR was reported for Fayoumi breed during 9-20 weeks of age.
Carcass characteristics
Significant differences in the carcass traits were observed among the local, exotic and their crossbred chicken. The exotic RIR and crossbred NNRIR had higher dressing percentage, breast, thigh and drumstick yields while lowest carcass traits were recorded for NN. All experimental birds in our trial were kept in a similar environmental condition in raised pens and were fed the same diet. It is thus expected that any differences that were observed between the different genotypes, for carcass traits and chemical composition, are the result of genetic differences.
Our results are in line with Azharul et al. (2005), who reported higher dressing percentage in crossbred of RIR × Fayoumi as compared with Fayoumi breed. Some studies showed that crossbreds from NN with RIR, White leghorn or Fayoumi resulted in improved dressed and total meat yield in comparison with exotic or NN chickens (Haque et al., 1999; Haque and Howlider, 2000).
Meat composition
The chemical composition was significantly affected by genotypes used in this trial. The exotic RIR and crossbred NNRIR had significantly higher CP and dry matter in thigh and drumstick meat as compared to the NN, while the NN had higher fat content in thigh and drumstick meat. Faster growing chickens tends to have a higher proportion of larger white muscle fibers than slower growing chickens which have more red muscle fibers that are smaller in diameter (Dransfield and Sosnicki, 1999), which may be responsible for the higher CP in RIR and crossbred NNRIR.
The results obtained in this trial can be compared well with those obtained by Packard (2014) and Wattanachant (2008), who concluded that genetics, age and rearing system influence muscle chemical composition. Findings of the current study regarding dry matter and protein content in the NN chicken are similar to the results of Wattanachant (2008). Contrary to our findings, Packard (2014) recorded highest thigh and drumstick CP for NN as compared to a hybrid between a Cobb 500 broiler and African native breed (Potchefstroom Koekoek).
Immune competence
It is indicated that CMI response is important for defense against intracellular bacterium (Lillehoj and Okamura, 2003; Barua and Yoshimura, 2004). The PHA skin response test is a measure of inflammatory swelling in response to mitogenic PHA derived from plant or bacterial protein called lectins. This test examines T-cell functions by attaching PHA to special receptors on their surface to induce lymphokines secretion that in turn increases permeability of blood vessels. As a result, a number of leukocytes and fluid ooze out to the site of injection causing increase in skin thickness that indicates T-cell-mediated immune response (Grasman, 2010). Our results showed significantly higher response for NN and crossbred NNRIR against PHA-P while lowest for exotic RIR chickens. These results are backed by Abou-Emera et al. (2017), Mahrous (2008), El-Safty (2006), Fathi (2005) and Patra et al. (2004) who observed significantly higher CMI response in purebred and crossbred NN genotypes compared to their normal feathered counterparts. In contrast to our findings, Rajkumar et al. (2011) observed non-significant immune response in NN and its counterparts against PHA-P in both winter and summer season. Alvarez (2002) noticed higher CMI response in heterozygous NN genotype than either normal feathered (nana) genotypes or pure-bred NN genotypes.
Sheep red blood cells (SRBC) have been used as antigen for humoral immune response in this study because of their natural multi– factor, and ability to activate chicken immune response without any adverse effects (Grasman, 2010). Our study revealed significant total antibody titre against SRBCs for indigenous NN followed by crossbred NNRIR (Figure 2). Our results are favored by El-Safty (2006), Fathi et al. (2008), Patra et al. (2004); Mahrous (2008) and Rajkumar et al. (2010b), who noticed significantly higher anti-SRBC titres for NN birds and their crosses as compared to other normal feathered birds. Fathi et al. (2008) reported significantly higher anti-SRBC titers for NN birds at 7 and 14 days post-secondary injection.
Unlike T-cells, phagocytic immune response commences soon after exposure to foreign antigens (El-Safty, 2012). Phagocytosis in response to injectable India ink in representative birds of different genetic groups showed significantly low level of carbon in indigenous NN birds and their crossbred NNRIR as compared to exotic RIR chickens. These results are favored by Mahrous (2008) who indicated significantly better carbon clearance for NN, frizzle and NN-frizzled chicks while poor clearance for normally feathered genotype and concluded that presence of NN, Frizzle or both genes combine are resistant to bacterial, viral and parasitic infections. Similar results have been reported in crossbreds of NN and normal feathered birds in Egypt by Nazmi (2006). Phagocytosis may be influenced by B complex (Cheng and Lamont, 1988) and birds with better phagocytic response can resist bacterial, viral, and parasitic infections (Qureshi et al., 2000). On the other hand, Haunshi et al. (2002) noticed non-significant differences in phagocytic response of NN and normally feathered genotypes.
CONCLUSiONS AND RECOMMENDATİONS
Overall significant differences in all performance and immune competence traits were found among indigenous NN, exotic RIR and their crossbred NNRIR chickens. Growth performance of NNRIR crossbred chickens was higher than indigenous NN chickens and had better immune responses than exotic RIR chickens. It was concluded that crossbreds have potential for improving quantitative traits of indigenous chickens in the tropics without compromising their ability to cope with harsh environmental conditions, ultimately resulting in better economic outcomes and converting subsistence rural poultry farming into profitable ventures.
Novelty Statement
Incorporating the Naked Neck gene in breeding programs is an important aspect of poultry breeding for anticipated global warming and climate change In this study, Naked Neck chickens were crossed with exotic Rhode Island Red chickens to improve their growth potential without compromising their heat tolerance potential in the face of global warming and climate change (developing climate-smart breeds to mitigate the anticipated effects of climate change).
Author’s Contribution
The authors confirm contribution to the paper as follows: Study conception and design: M. Alam, N. Chand, S. Khan; data collection: M. Alam; analysis and interpretation of results: M. Alam, N. Chand, S.M. Suhail; draft manuscript preparation: M. Alam. All authors reviewed the results and approved the final version of the manuscript.
Conflict of interest
The authors have declared no conflict of interest.
REFERENCES