Journal of Animal Health and Production
Research Article
Probiotic Properties of Bacillus subtilis Isolated from Dried Anchovies (Stolephorus indicus) and Evaluating its Antimicrobial, Antibiofilm and Growth-Enhancing Potential in Danio rerio
Thejaswi Bhandary, Riyaz Ali.L, Kuppusamy Alagesan Paari*
Department of Life Sciences, CHRIST (Deemed to be University), Hosur main road, Bangalore, Karnataka, India 560029.
Abstract | The study was aimed at isolating and characterising a potential probiotic bacterium from dried anchovies (Stolephorus indicus) and evaluating its antibacterial, antibiofilm and growth enhancing potential in Danio rerio. The isolate was identified as Bacillus subtilis using 16S rRNA sequencing and phylogenetic analysis. Probiotic properties were characterised based on the ability of the isolated strain to survive in simulated gastric juice and trypsin. Isolated strain was further subjected to varying pH, temperature, different concentrations of organic solvents to evaluate its potential to tolerate stress. Biofilm inhibition against Vibrio harveyi (31.5±4.6%), Escherichia coli (28.8±4.2 %), Pseudomonas aeruginosa (34.8±3.1%) and Staphylococcus aureus (34.4±3.75%) was noted. The study showed that the isolate improved the survival rate of Danio rerio against Vibrio harveyi and Escherichia coli. The weight (12.77±0.06) and length (11.413±0.18) gain percentage was numerically (p> 0.05) improved in probiotic supplemented groups as compared to control. The use of probiotics from non-conventional sources can improve the diversity of the available probiotics for aquaculture practices
Keywords | Bacillus subtilis, Probiotic, Aquaculture, Anti-bacterial, Anti-biofilm, Growth enhancement
Received | March 14, 2021; Accepted | April 15, 2021; Published | June 15, 2021
*Correspondence | Kuppusamy Alagesan Pari, Department of Life Sciences, CHRIST (Deemed to be University), Hosur main road, Bangalore, Karnataka, India 560029; Email: paari.ka@christuniversity.in
Citation | Bhandary T, Ali L Riyaz, Paari KA (2021). Probiotic properties of bacillus subtilisisolated from dried anchovies (stolephorus indicus) and evaluating its antimicrobial, antibiofilm and growth-enhancing potential in danio rerio. J. Anim. Health Prod. 9(3): 205-212.
DOI | http://dx.doi.org/10.17582/journal.jahp/2021/9.3.205.212
ISSN | 2308-2801
Copyright © 2021 Ali L et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Among the various food production sectors, aquaculture is the most rapidly growing. The average annual increase in global consumption of fish (3.2%) outpaced the average growth in population (1.6%) between 1961 and 2016 (FAO, 2018). Coupled with this growth are the various concerns related to the safety and quality of the fish due to overproduction to meet the needs of billions of people around the globe. One of the major constraints in the field of aquaculture is the surge in disease outbreaks. Antibiotics have been the go-to option to combat disease outbreaks (Satish et al., 2011). Over-prescription of antibiotics in clinical setups and in the food industry to the emergence of antibiotic resistant microorganisms such as Staphylococcus aureus, Enterococci, and Streptococcus pneumoniae (Hols et al., 2019). Due to the growing concerns about the use and abuse of antibiotics, alternatives such as probiotics are emerging as a safer and beneficial alternative (Satish et al., 2011).
A probiotic is a cultured product or live microbial feed supplement, which beneficially affects the host by improving the intestinal microflora (Fuller, 1989). Probiotics are mostly associated with the gastrointestinal tract, where they are antagonistic to pathogenic bacteria (Hoseinifar et al., 2018). Some of the mechanisms by which the probiotics confer beneficial effects to the host are by causing the exclusion of pathogenic microorganisms (Gayathri and Rashmi, 2016), improving the host immune response by producing antibacterial substances such as antibiotics, bacteriocins (Gaspar et al., 2018; Hoseinifar et al., 2018), siderophores (Panda et al., 2017), alteration of pH by organic acid production and by the production of hydrogen peroxide under aerobic growth conditions (Pavlova, 2020). Probiotics are also known to reduce mutagenesis by directly binding to mutagenic compounds thereby reducing their absorption in the intestine (Orrhage et al., 1994), and reduce cholesterol production through the production of short-chain fatty acids (Pereira and Gibson, 2002).
Researchers have relied on different sources for the isolation of potential probiotic bacteria. The conventional sources are dairy products and the gastrointestinal tract of healthy humans. However, research conducted over the years has highlighted the use of alternate sources such as the gastrointestinal tract of other animals, traditional fermented vegetables, fruits, and dried fish (Zielińska and Kolożyn, 2018; Singh et al., 2012). One example of such an unconventional source, dry fish, has been gaining popularity. There has been an increase in the production of dry fish with about 17% of the total catch in Indian fisheries being used for the production of dry fishes (Bharda et al., 2017). Mackerel, tuna, oil sardines, lesser sardines, silver bellies, mullets, ribbon fishes, and anchovies are some of the species commonly subjected to dry fish production in India (Siriskar et al., 2013, Logesh et al., 2012). Dried anchovies (Stolephorus indicus) form an important component of purse-seine fishery of the Indian coast that are found mostly along the coastal areas of India and are widely consumed as salted or in dried form (Siriskar et al., 2013). Traditionally dried anchovies are a potentially unique source of probiotic bacteria (Alkalbani, 2019). Thus, the current study was conducted with the following objectives: (1) to isolate potential probiotic bacteria from dried anchovy fish and characterize their probiotic properties and (2) to decipher its use in combating pathogens, enhancing the growth and survival rates in fishes.
MATERIALS AND METHODS
Isolation, Identification and Characterisation
The isolation source for probiotics was dried Anchovy fish (Stolephorus indicus). MRS (De Man-Rogosa-Sharpe) media was used for isolating the culture. The isolate was identified using 16SrRNA sequencing using forward (16SrRNA-F) and reverse (16SrRNA-R) primers by BDT v3.1 cycle sequencing kit on ABI 3730xl Genetic Analyzer. Gram’s staining, IMViC test, motility and catalase tests were performed as preliminary evaluation (Hoque et al., 2010). The isolate was screened for its antimicrobial (Kavitha et al., 2018) and haemolytic activity (Halder et al., 2017) before further characterisation.
pH, temperature and tolerance test
Characterisation was done according to the protocol of Yadav et al. (2016) with slight modifications. The isolate was exposed to varying pH (2-10), temperature (15oC-55 oC), sodium chloride concentrations (2 %-10%) and solvent concentrations (0.2% - 0.6%). After incubation for 24 h, the culture was plated onto MRS agar plates and the Log (CFU•mL-1) was calculated (Zheng et al., 2013).
Artificial gastric juice tolerance and trypsin tolerance test:
The test was performed according to the protocol described by Corcoran et al. (2005) with slight modifications. The composition of the artificial gastric juice was Glucose (0.35 g), NaCl (0.20 g), KH2PO4 (0.06 g), CaCl2 (0.01 g), KCl (0.03 g) and Pepsin (0.03 g) per 100 mL. All components of gastric juice were added as stock in the media prior to inoculation of culture. An overnight culture of the isolate was inoculated into artificial gastric juice and incubated at 37 oC for time duration of 2, 4 and 6h. Similarly, the isolate was subjected to varying concentrations (0.2% - 0.6 %) of trypsin. The isolate was treated with trypsin for a period of 6h. The cell viability was expressed in Log (CFU•mL-1) after plating onto MRS agar for 24 h at 37oC.
In vivo challenge and growth studies
Challenge studies were performed according to the protocol of Wang et al. (2008) with slight modifications. A total of 180 healthy Zebrafish (Danio rerio) weighing (0.1-0.15 g) were segregated into triplicates in the following groups; Group treated with saline, group treated with probiotic only, group treated with pathogens (Vibrio harveyi and Escherichia coli) and a final group constituting pathogens along with the isolate. The survival rate in D. rerio was determined by supplementing 6 Log (CFU•mL-1) of the isolate against Escherichia coli and Vibrio harveyi (6 Log (CFU•mL-1). Parameters such as feed conversion ratio, weight gain percentage and length gain percentage were monitored for a period of 15 days.
Antibiofilm Activity
Escherichia coli, Pseudomonas aeruginosa, Vibrio harveyi and Staphylococcus aureus were allowed to form biofilm in a microtiter plate. The antibiofilm activity was measured using a microplate reader (iMark™ Microplate Absorbance Reader, Biorad) set at 450 nm and the percentage inhibition was calculated (Costa et al., 2018).
Statistical Analysis
The results are presented as Mean±SD of triplicates. The various parameters analyzed were subjected to statistical analysis using one way ANOVA and independent t-test with p <0.05 being considered significant.
Table 1: General characteristics of the isolated Bacillus subtilis
|
Colony morphology
Gram’s staining and Shape |
White,round,opaque, medium and smooth Gram positive, bacilli |
(Lu et al., 2018)
(Seenivasan et al., 2012) |
| Catalase test | + | (Lu et al., 2018) |
| Motility test | Motile | (Liu et al., 2009) |
| IMViC test* | +--+** | (Seenivasan et al., 2012) |
| Haemolysis assay | Gamma Hemolytic | (Zulkhairi et al., 2019) |
| Milk coagulation efficacy | - | (Wu et al. 2013) |
| Growth at 0.6% trypsin | ++ | (Nithya and Halami, 2012) |
| Antimicrobial well diffusion test | ++ | (Kavitha et al. 2018) |
| Organic acid production | - | (Hoque et al. 2010) |
*Indole, Methyl red, Voges-Proskauer and Simmon citrate test.
**Consecutive values according to IMViC test
Results and Discussion
The isolate was identified as Bacillus subtilis using 16S rRNA sequencing and the partial sequence was deposited in NCBI (Accession number -MN960600). The evolutionary history was inferred using the Maximum Likelihood method and Tamura-Nei model and a phylogenetic tree was constructed (Tamura and Nei, 1993) (Figure 1) and the closest relative was identified as Bacillus subtilis. The genus Bacillus comprises a diverse group of organisms that offer a wide range of nutritional, physiological and metabolic diversity (Sen et al., 2015). B. subtilis has been widely used due to its antimicrobial activity to tackle fish pathogens in commercial aquaculture (Wu et al., 2018; Kong et al., 2017). The isolate in the study was biochemically characterised (Table 1) and screened for its anti-microbial activity against pathogens like E. coli and V. harveyi.
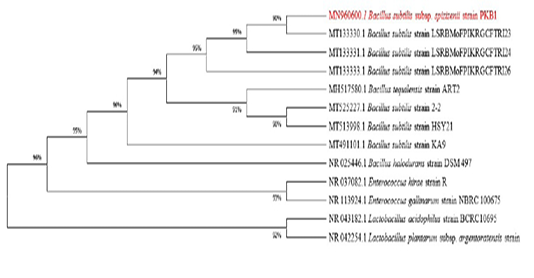
Figure 1: Depicts MEGA-X phylogenetic analysis of Bacillus sp. isolate. The tree with the highest log likelihood (-4108.98) is shown. Initial tree(s) for the heuristic search were obtained by applying Neighbour-Join and BioNJ algorithms to a matrix of pairwise distances estimated using the Maximum Composite Likelihood (MCL) method and then selecting the topology with superior log likelihood value. The tree was drawn to scale, with branch lengths measured in the number of substitutions per site.
The isolate in this study was able to tolerate temperature up to 45oC and the growth subsided beyond 45oC (Figure 2). Minimal or no growth was reported at lower temperature ranges below 25oC (data not shown). Probiotics can rarely tolerate temperature changes beyond 50oC (Ding and Shah, 2007). Thermal resistance in probiotics is key in determining survival ability since fluctuations in temperature in external or internal environments can lead to cell death (Champagne et al., 1993).
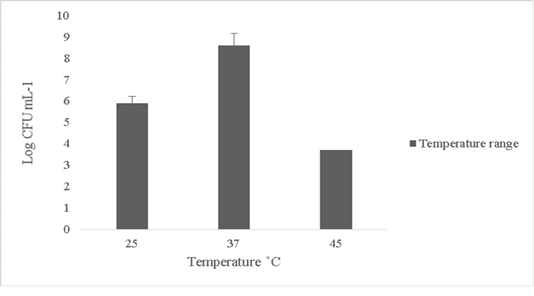
Figure 2: Thermal tolerance of Bacillus subtilis-MN960600 on upshifts and downshifts from 37˚C *Error bars indicate the standard deviation, the values are expressed as Mean±SD of triplicates
The isolate showed maximum growth at pH 6-7 with 8.1±0.4 Log CFU·mL-1 and growth was 7.6±0.1 Log CFU·mL-1 at pH 4. No growth was observed at pH 2 and the growth dropped by over 1.1±0.2 Log CFU·mL-1 at pH ranges of 8-10 (Figure 3). B. subtilis is able to tolerate varied ranges of pH and the optimum pH showing maximum activity ranges between 5 to 7 (Cotter and Hill, 2003). Bacillus sp. are known to tolerate acidic conditions (pH 2.5) and such candidates are suitable to be employed as starter cultures as they are able to survive the internal gastric environment and possess lesser or equal enzymatic activities (Krulwich et al., 1985; Jeon et al., 2017). The ability of the isolate to tolerate wide ranges of pH fluctuations exerts its antimicrobial activity in the extreme pathogenic environment.
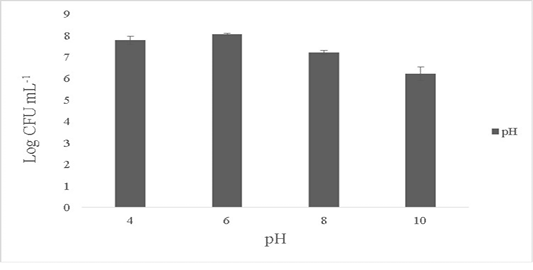
Figure 3: Effect of pH on the survival of the Bacillus subtilis-MN960600. *The data are means of triplicate experiments and error bars indicate standard deviations.
Another probiotic characteristic is the ability to survive in the internal gastric environment (Ding and Shah, 2007). The isolate was checked for its ability to survive in simulated artificial gastric juice for time duration of 2, 4 and 6h mimicking the estimated time to be spent in the gastrointestinal tract (Figure 4). The final growth observed after 6h was 7.0±0.31 Log (CFU·mL-1).
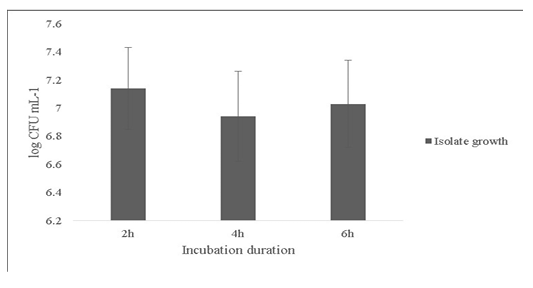
Figure 4: Survival of the Bacillus subtilis-MN960600 in artificial gastric juice at durations 2h, 4h and 6h. *Error bars indicate the standard deviation; the values are expressed as Mean±SD of triplicates
Salt tolerance can influence the water and enzymatic activity of bacterial strains and confers high osmotolerance to withstand excessive external osmotic pressure (Adnan and Tan, 2007). Bacillus sp. is highly tolerant to salt concentrations ranging from 2% -10% (Ragul et al., 2017). At 2% concentration, growth was 7.98±0.56 Log (CFU•mL-1) and at 10% concentration, growth was found to be 6.98±0.29 Log (CFU•mL-1) (Figure 5). An important facet for a probiotic is its ability to survive the action of toxic metabolites like phenol and organic solvents like toluene. Primary phenols are the by-products of the digestive process. Some probiotics retain added bacteriostatic activity by metabolising aromatic amino acids into phenols (Kumar et al., 2019; Suskovic et al., 1997). In this study, isolate was able to tolerate phenol and toluene at 0.6% concentrations [7.8±0.08 Log (CFU•mL-1) and 8.5±0.09 Log (CFU•mL-1)] respectively (Figure 6).
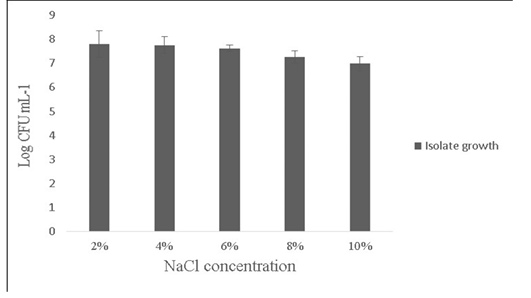
Figure 5: Showing the effect of NaCl (2-10%) tolerance on the growth of Bacillus subtilis -MN960600. *Error bars indicate the standard deviation; the values are expressed as Mean±SD of triplicates
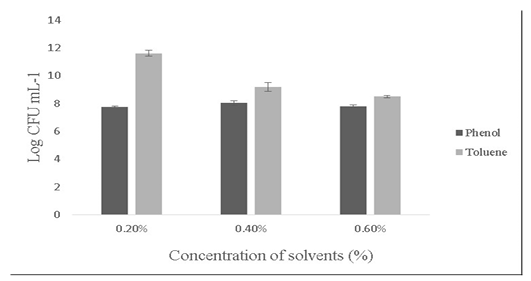
Figure 6: Showing the effect of solvents (Phenol and toluene) on the growth of Bacillus subtilis-MN960600. *Error bars indicate the standard deviation; the values are expressed as Mean±SD of triplicates
The anti-biofilm forming activity of the isolate was evaluated against Escherichia coli, Vibrio harveyi, Pseudomonas aeruginosa and Staphylococcus aureus and was found to be
28.8±4.2%, 31.5±4.6%, 34.8±3.1% and 34.4±3.75% respectively (Figure 7). Bacillus sp. possess remarkable antimicrobial properties which can be attributed to the production of bacteriocins like fengycin, surfactin, bacilysin, butirosin, macrolactin, bacillaene, difficidin, bacillibactin, lanthipeptides and LCI. Antimicrobial peptides are also produced by genes like LCI, YFGAP and hGAPDH and gene clusters for secondary metabolites (Wu et al., 2018). Bacterial motility is crucial for colonization processes of pathogens. Bacillus sp. isolated from a marine niche has been used to decrease Vibrio alginolyticus motility and thereby reduce its pathogenicity against Danio rerio. The potential lead compounds from Bacillus sp. targeting bacterial motility and biofilm inhibition are encouraged to be used in aquaculture. Bacillus sp. have a low potential for eliciting antibiotic resistance since they are not involved in horizontal gene transfer and are unlikely to acquire genes pertaining to antibiotic resistance (Xiu et al., 2017).
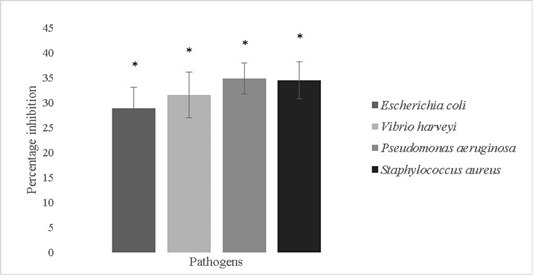
Figure 7: Antibiofilm activity of Bacillus subtilis-MN960600 against Escherichia coli, Vibrio harveyi, Pseudomonas aeruginosa and Staphylococcus aureus. **The error bars indicate standard deviation and statistical significance indicated by * (P<0.05)
Feed conversion ratio (FCR) was lesser compared to the control sample indicating a potential of improved nutrition absorbability. Similarly, other growth parameters like length gain and weight gain percentages were found to be better in probiotic supplemented samples but no significant difference in the growth parameters between the control and treatment groups was noted (Table 2).
Table 2: Growth parameters in Danio rerio upon treatment with Bacillus subtilis isolate
| Parameters | Control | Probiotic | P- value |
|
Feed conversion ratio |
0.32±0.07 | 0.27±0.09* | 0.24 |
|
Weight gain percentage |
12.3±0.08 | 12.77±0.06* | 0.18 |
|
Length gain percentage |
8.9±0.32 | 11.413±0.18* | 0.43 |
Note: The values are Mean±SD of triplicates, the statistical significance was verified using independent student t-test and the non-significant (p> 0.05) difference between rows are highlighted using *
Bacillus sp. have been widely used as growth promoters and contribute for improved survival rate (Gatesoupe, 1999). In this study, Bacillus sp. was used to assess the survival of Danio rerio against fish pathogens as described in the survivorship cure (Figure 8). Bacillus spp. improve food absorption by enhancing protease levels, providing resistance against pathogens like V. harveyi and luminescent vibrio spp. in both fishes and penieds (Irianto and Austin, 2002; Moriarty, 1999; Lakshmi et al., 2013). Bacillus subtilis stimulates mRNA expression of both pro-inflammatory and anti-inflammatory cytokines in dendritic cells of grass carp via cytokine-related pathways (Zhou et al., 2019). Bacillus amyloliquefaciens G1 has been used against a wide spectrum of pathogenic Aeromonas hydrophila strains by having a protective antimicrobial action in Anguilla anguilla (Lu et al., 2011). Furthermore, combination cultures of Bacillus sp. have been used to enhance innate immune response in Perca flavescens, thereby contributing to lesser risk of disease susceptibility. Similarly, a combination mixture of Bacillus subtilis and Bacillus licheniformis (BioPlus2B) has been used against Yersinia ruckeri infected trout to improve their survival rate (Raida et al., 2003; Salim and Reda, 2015). Bacillus subtilis has also been used against A. hydrophila, Edwardsiella ictaluri, Streptococcus sp. in Cirrhinus cirrhosus, Oncorhynchus mykiss, Ictalurus punctatus, Pangasianodon hypophthalmus, Epinephelus summana and Oreochromis mossambicus to improve their survival rate
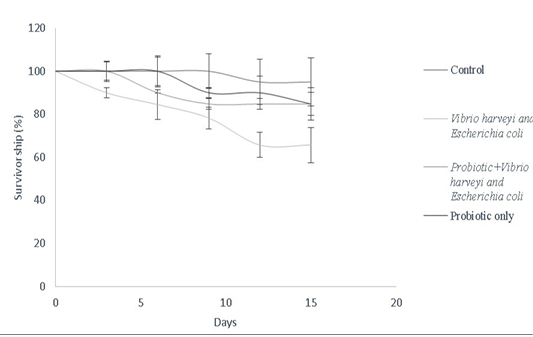
Figure 8: Survivorship curve of D. rerio depicting the effect of B.subtilis- MN960600 supplementation against pathogen challenge in a total duration of 15 days (Control, Vibrio harveyi and Escherichia coli, probiotic+Vibrio harveyi and Escherichia coli and probiotic only). Error bars indicate the standard deviation; the values are expressed as Mean±SD of triplicates
(Kumar et al., 2008; Newaj et al., 2007; Ran et al., 2012). Bacillus licheniformis, Bacillus pumilus and Bacillus circulans have been used against Streptococcus agalactiae and A. hydrophila in Catla catla, Paralichthys olivaceus and Oreochromis niloticus to improve immune health status and disease resistance (Aly et al., 2008; Babdyopadhyaya and Mohapatra, 2009; Cha et al., 2013; Han et al., 2015). Bacillus sp. S11, NL 110 and B. coagulans have been supplemented to Penaeus monodon, Ictalurus punctatus, Macrobrachium rosenbergii, Cyprinus carpio Litopenaeus vannamei and Fenneropenaeus indicus to improve nutrient digestibility (Queiroz and Boyd, 1998; Rahiman et al., 2010; Rengpipat et al., 1998; Lin et al., 2012; Heizhao et al., 2004). B. subtilis, L. acidophilus, S. cerevisiae have been used in Paralichthys olivaceus to improve stress tolerance (Taoka et al., 2006). Bacillus subtilis has also been used in Poecilia reticulata, Xiphophorus maculatus for reproductive enhancement (Ghosh et al., 2007).
Conclusion
Our study revealed the promising effect of Bacillus subtilis on growth and disease resistance of Danio rerio. Dietary supplementation of Bacillus subtilis as an eco-friendly alternative to antibiotics gained enormous attention in the fisheries sector. Results of our study confirmed the biocontrol potential of Bacillus subtilis against aquaculture pathogens. Widespread research should be conceded out to analyze the species specific interaction of the host and Bacillus subtilis.
Acknowledgements
The authors acknowledge the support received from the Department of Life Sciences and Centre for Research, CHRIST (Deemed to be University) for the financial aid through MRP. (MRPDSC-1936).
Conflict of Interest
The authors declare no conflict of interest
Author contribution
Thejaswi Bhandary was involved in the experimental design, data collection, data analysis and revised this manuscript. Riyaz Ali.L was involved in experimental design, data analysis and drafted this manuscript. Kuppusamy Alagesan Paari conceptualized the idea, involved in funding and revised this manuscript. All authors read and approved this manuscript.
References





