Journal of Animal Health and Production
Research Article
Effects of Phytogenic Supplementation on Productive and Economic Performance in Broilers
Sara Esam Shahin1*, Doaa Ibrahim2, Mohamed Badawi2
1Veterinary Economics and Farm Management, Department of animal wealth development, Faculty of Veterinary Medicine, Zagazig University, 44519 Zagazig, Egypt; 2Department of Nutrition and Clinical Nutrition, Faculty of Veterinary Medicine, Zagazig University, 44519 Zagazig, Egypt.
Abstract | Two hundred Ross 308 male one-day-old broiler chicks were applied to investigate the effect of phytogenic feed additive (PFA), characterized by phenolic terpenes, hydroxphenylpropenes flavones, phenolic acids, and aldehydes, on growth performance, some serum biochemical parameters, the gene expression profile of anti-oxidant enzymes, and economic efficiency. They were randomly assigned to one of four experimental treatments with five replicates per treatment (50 birds/treatment; 10 birds/replicate). Birds were fed with diets supplemented with 0 (control), 0.05, 0.10 and 0.20% PFA following a 3 phase (i.e., starter, grower and finisher) feeding program for 42 days. PFA supplementation significantly improved (P<0.05) the different growth performance parameters including weight gain, feed intake and feed conversion ratio. Furthermore, dietary PFA significantly reduced (P<0.05) the serum total cholesterol, and LDL-cholesterol levels as compared to the control diet. The highest gross margin, benefit-cost ratio, economic efficiency, and lowest cost/kg were recorded in the broiler group fed on 0.20% PFA. Also, PFA dietary inclusion at 0.20% significantly augmented (P<0.05) the gene expression of GST/GAPDH, SOD/GAPDH and Catalase/GAPDH genes, compared with the un-supplemented controls. Birds fed diets containing 0.20% PFA exhibited the highest growth, economic efficiency, and gene expression. It could be concluded that supplementation with PFA in broiler chicken diets has beneficial effect on growth performance and biochemical parameters, and also improved economic efficiency through maximizing both returns and gross margin as well as gene expression profile of anti-oxidant enzymes.
Keywords | Broiler, Phytogenic, Performance, Biochemical, Gene expression, Economics
Editor | Asghar Ali Kamboh, Sindh Agriculture University, Tandojam, Pakistan.
Received | November 03, 2020; Accepted | November 23, 2020; Published | December 27, 2020
*Correspondence | Sara Esam Shahin, Veterinary Economics and Farm Management, Department of animal wealth development, Faculty of Veterinary Medicine, Zagazig University, 44519 Zagazig, Egypt; Email: saraesam2011@gmail.com
Citation | Shahin SE, Ibrahim D, Badawi M (2020). Effects of phytogenic supplementation on productive and economic performance in broilers. J. Anim. Health Prod. 9(s1): 42-49.
DOI | http://dx.doi.org/10.17582/journal.jahp/2020/9.s1.42.49
ISSN | 2308-2801
Copyright © 2020 Shahin et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Antibiotic growth promoters are using in animal production to promote growth and feed conversion since decades, however, now they were banned in many parts of the world due to several reported dangerous side effects on animals, poultry, and human (El-Ashram and Hamady, 2020). Searching an inexpensive, safe, and acceptable substitute to replace antibiotics is todays’ top research trend. The application of growth promotors has added a huge benefit to the poultry industry, sometimes doubling the net weight of the birds without any additional dietary supplementation. Recently, the usage of phytogenic feed additives (PFAs) in poultry disease controlling and growth promotion has established novel interest, due to their cost-effective applications and minimal side effects (Gheisar and Kim, 2018).
Herbs or Phytobiotics applied in classic medication are also known as PFAs (Franz et al., 2010). They include a vast extent of plant-derived products like herbs (non-persistent non-woody, and flowering plants), spices (herbs with a strong taste or smell generally used in human food), oleoresins (extracts originated from non-aqueous solvents), and essential oils (volatile lipophilic substances) (Windisch et al., 2008). They could be used as alternatives to antibiotics (Rochfort et al., 2008) due to their commonly shown growth-promoting, anti-microbial (Gheisar et al., 2015), anti-oxidant (Miguel, 2010), and anti-inflammatory effects (Giannenas et al., 2013) as well as improve palatability and gut function (Brenes and Roura, 2010). The anti-oxidative role of PFAs can positively impact the persistence of animal diet and raise animal’s products storage time and quality (Giannenas et al., 2013). The anti-carcinogenic impacts of PFA are based on the inducement of genes with an ‘antioxidant response element’ (ARE) in their DNA promoter. ARE consist of mRNA genes expression involve anti-oxidant enzymes like glutathione-S-transferases (GST), superoxide dismutase (SOD), catalase, and others/ glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Mueller et al., 2012).
In general, the published papers denote the positive impacts of PFAs on poultry production performance. Because of the conflicts in published results, further investigations and research are still substantial to demonstrate different nutritional characteristics of PFAs. As the available information about their aspects of the use and modes of the mechanism are still rather limited (Gheisar and Kim, 2018). The main aim of this trial was, therefore, to determine the effect of dietary supplementation of PFAs on growth performance, some serum biochemical parameters, gene expression, and economic evaluation of broiler (Ross 308) chickens.
MATERIALS AND METHODS
Animals and feeding
All research strategies have been accomplished in compliance with local experimental animal care regulation and approved by the institutional ethics committee, Faculty of Veterinary Medicine Zagazig University, Egypt.
Male one-day-old Ross 308 chicks (n= 200), weighing 40±1 g, were allocated to floor pens, temperature, light schedule, and vaccination protocol meet the regular guidelines. The birds were randomly allocated to 4 equal treatments with 5 replicates/treatment (50 chicks/ pen; 10 chicks/replicate). Phytogenic feed additive (PFA) product (Anco FIT®) containing phenolic terpenes, hydroxphenylpropenes flavones, phenolic acids, and aldehydes was purchased from the Nagi Awad group produced by ADM Company. Table 1 showed experimental treatments containing diets supplemented with PFA at rate 0 (control treatment), 0.5 (treatment 2), 1.00 (treatment 3), and 2.00 gm/kg diet (treatment 4) for 42 days and were formulated to supply Ross 308 requirements (Management guide for Ross 308, 2019). The water and feed were available ad-libitum.
Table 1: Ingredients of the experimental diets and formulation.
| Ingredients % |
Stage of growth |
||
| Starter diets (1-10 days) | Grower diets (11-22 days) |
Finisher diets (23-42 days) |
|
| Yellow corn | 56.055 | 61.405 | 63.405 |
| Soybean meal, 48% | 32.60 | 28.64 | 29.00 |
| Corn gluten meal, 60% | 4.60 | 3.00 | 0.00 |
| Soybean oil | 1.92 | 2.5 | 3.70 |
| Calcium carbonate | 0.59 | 0.56 | 0.48 |
| Ca. dibasic phosphate | 2.73 | 2.49 | 2.08 |
| Common salt | 0.35 | 0.25 | 0.27 |
| Sodium bicarbonate | 0.25 | 0.25 | 0.20 |
| Mineral mixture** | 0.072 | 0.074 | 0.074 |
| Vitamin mixture* | 0.033 | 0.031 | 0.031 |
| Choline chloride, 60% | 0.07 | 0.06 | 0.05 |
| L-Lysine, Hcl, 79.80% | 0.35 | 0.33 | 0.26 |
| L- Methionine, 100% | 0.29 | 0.27 | 0.30 |
| L-Threonine, 99% | 0.09 | 0.09 | 0.10 |
|
Mycofix select |
0.05 | 0.05 | 0.05 |
| Calculated composition | |||
| ME, Kcal/Kg | 3035.75 | 3110.69 | 3184.79 |
| CP, % | 23.02 | 20.58 | 19.07 |
| EE, % | 4.62 | 5.29 | 6.47 |
| CF, % | 2.56 | 2.50 | 2.53 |
| Ca, % | 0.96 | 0.88 | 0.76 |
| Available phosphorus, % | 0.48 | 0.44 | 0.38 |
| Lysine, % | 1.41 | 1.27 | 1.20 |
| Methionine, % | 0.68 | 0.62 | 0.61 |
| Analyzed composition | |||
| DM, % | 9 | ||
| CP, % | 2 | ||
| EE, % | 4 | ||
| CF, % | 2.36 | 2.17 | 2.30 |
| Ash, % | 3.60 | 3.55 | 3.57 |
Each 3 kg of premix contain. * Vitamin mixture: vit. A (10, 000000 IU), vit. D3 (2, 000000 IU), vit. E (10 g), vit. k3 (1000 mg), vit. B1(1000 mg), vit. B2 (5 g), vit. B6 (1.5 g), pantothenic acid (10 g), vit. B12 (10 mg), niacin (30 g), folic acid (1000 mg) and biotin (50 g). ** Mineral mixture: Fe (30 g), Mn (60 g), Cu (4 g), I (300 mg), Co (100 mg), Se (100 mg) and Zn (50 g).
Final live body weight was recorded and body weight gain (BWG), feed intake, and feed conversion ratios (FCR) were calculated at the end of the experiment (Wegner, 1992).
Serum biochemistry
The serum lipid profile: total cholesterol (TC; Pisani et al., 1995), triglycerides (TAG; Stein and Myers, 1995), high-density lipoprotein-cholesterol (HDL-C; Nitschke and Tall, 2005), low and very density lipoprotein-cholesterol (LDL-C; VLDL-C; Sonntag and Scholer, 2001), and liver and kidney function indicators viz., alanine aminotransferase/aspartate aminotransferase (ALT/AST; Young, 2001), urea and creatinine (Henry, 1974) were analyzed using commercial diagnostic kits (Spinreact., Santa Coloma, Spain). For all analyses, blood was collected from randomly selected birds (five per treatment) at the end of trial (42 d).
mRNA expression of Antioxidant enzymes
At the end of the trial, 5 birds/treatment were randomly slaughtered and liver tissues were collected aseptically. The total RNA extracted from the tissues using the RNeasy Mini Kit (Qiagen, Cat. No. 74104) as per the instructions of manufacturer. The extracted RNA was measured using the NanoDrop® ND-1000 Spectrophotometer (NanoDrop Technologies, Wilmington, NC, USA). The first-strand cDNA synthesized using RevertAidTM H Minus kits (Fermentas Life Science, Pittsburgh, PA, USA). One microliter of this cDNA was mixed with 12.5 microliter of 2x SYBR® Green PCR mix with ROX from BioRad, 5.5 microliter of RNase free water, and 0.5 microliter (10 11 pmol/μL) of each forward and reverse primer for the selected genes. The primers’ sequences of selected genes are:
GST/ GAPDH (glutathione peroxidase),
F- CGCCGAAGGTCTCGTTATT
R- TCCCTGGACGGACACTT,
SOD/ GAPDH (superoxide dismutase),
F- ATGCGAAGTCTTCCACTGTC
R-ATGCGAAGTCTTCCACTGTC,
Catalase/GAPDH
F- CCCAGCTCTTCATCCAGAAAC
R-GCCTCCGCATTGTACTTCTT,
The real-time PCR amplification was done with Rotor-Gene Q2 plex (Qiagen Inc., Valencia, CA, USA) with the succeeding conditions; initial denaturation at 95°C for 10 minutes and 40 cycles at 95°C for 15 seconds, 60°C for 1 minute and 72°C for 30 seconds. Comparative fold changes in the expression of target genes measured in triplicate were assessed by the comparative 2−ΔΔCt method with the GAPDH gene as an internal control to normalize target gene expression levels (Schmittgen and Livak, 2008).
Economic analysis
The economic analysis was achieved by estimating the total expenses (TE) taking into account feed costs as well as the expenses of experimental bird, labor, and veterinary services. Feed cost/kg weight gain = FCR × cost of one kg diet. Total revenue (TR)= live body weight × price per kg. Gross margin (GM) = TR – TE. Benefit-cost ratio = GM / TE (Lundlhom, 2005). Economic efficiency = GM/ Feed costs (Omar et al., 2019).
Statistical analysis
The statistical analysis was carried out using the program SPSS/PCT, (Statistical Package for Social Sciences version 22.0) (IBM Corp., Armonk, NY, USA). Findings were reported by means±SEM (Standard Error of Mean). For statistical significance, the value of P < 0.05 was used. The statistical analysis was ANOVA (one-way variance analysis) test to measure the variations in the parameters of the efficient and economic performance of broiler according to different experimental diets. The Duncan multiple range test is also used.
RESULTS AND DISCUSSION
Production performance
The influence of PFA supplementation in Ross broiler diets on productive performance over the whole experimental period was shown in Table 2. The heaviest final BW, highest BWG, and lowest total feed consumed (P<0.05) were found in the broilers fed on a diet containing 0.20% PFA followed by those fed on a diet containing 0.05% PFA compared to other groups. In the same trend, the feed conversion ratio (FCR) was improved in groups fed on a diet containing 0.20% PFA followed by those fed on a diet containing 0.10% PFA while those fed on a diet containing control and 0.05% PFA recorded the highest FCR. Broilers that were fed on a diet containing 0.20% PFA had the heaviest final BW, highest BWG, and best FCR.
Serum biochemical profile
Table 3 presents the effects of the PFA on broiler blood parameters (TC, TAG, LDL-C, HDL-C, VLDL-C, ALT, AST, urea, and creatinine). The PFA didn’t affect the concentrations of TAG, HDL-C, VLDL-C, ALT, AST, urea, and creatinine activities. While the values of total cholesterol and LDL-C were dropped (P<0.05) in the broiler group fed on a diet containing 0.10% PFA when compared with other groups.
Economic performance
The effects of PFA addition in Ross diets on economic performance over the whole experimental period was exhibited in Table 4. The markedly highest (P<0.05) total return, gross margin, benefit-cost ratio, economic efficiency %, and lowest cost/kg BWG were found in the birds fed on a diet containing 0.20% PFA followed by those fed on a diet containing 0.10% PFA compared to other groups.
Table 2: The influence of dietary PFA inclusion on productive performance of broiler chicks (means± SEM).
| Trait studied | Experimental diets | P Value | |||
| Control | Phytogenic feed additive (PFA) | ||||
| 0.05% | 0.10% | 0.20% | |||
| Final body weight (kg) |
2.24± 0.009c |
2.23±0.007c |
2.42± 0.1b |
2.52± 0.01a |
0.001 |
| Body weight gain (kg) |
2.20± 0.02c |
2.19± 0.09c |
2.38± 0.07b |
2.48± 0.01a |
0.001 |
| Total feed intake (kg/bird) |
4.45± 0.019a |
4.46± 0.01a |
4.35± 0.013b |
4.30± 0.01c |
0.001 |
| feed conversion ratio |
2.02± 0.02a |
2.03±0.01a |
1.82± 0.01b |
1.73±0.09c |
0.001 |
abc Mean among the same row carrying different superscripts are markedly different at (P ≤ 0.05).
Table 3: The influence of dietary PFA inclusion on some blood metabolites of broiler chicks (means± SEM).
| Trait studied* | Experimental diets | P-Value | |||
| Control | Phytogenic feed additive (PFA) | ||||
| 0.05% | 0.10% | 0.20% | |||
| TC (mg/dl) |
106.67±3.38a |
101.33 ±1.45ab |
101.38±4.09ab |
94.00±0.57b |
0.001 |
| TG (mg/dl) | 98.33±6.00 | 91.67±5.36 | 102.00 ±2.08 | 95.33±2.4 | 0.90 |
| LDL-C (mg/dl) |
49.33±0.88 a |
39.23± 3.70ab |
39.60±2.60ab |
32.30±1.40b |
0.001 |
| HDL-C (mg/dl) | 37.69±2.02 | 43.67± 3.00 | 41.26± 5.04 | 42.60±1.02 | 0.10 |
| VLDL-C (mg/dl) | 19.67± 1.20 | 18.33± 1.07 | 20.40± 0.41 | 19.07± 0.48 | 0.25 |
| ALT (U/L) | 40.40± 2.76 | 39.93± 1.92 | 37.6± 1.22 | 41.30± 1.15 | 0.57 |
| AST (U/L) | 35.50± 1.80 | 32.86± 1.88 | 31.70± 0.75 | 31.83± 1.85 | 0.38 |
| Urea (mg/dl) | 12.56± 1.31 | 12.50±1.15 | 14.43±1.01 | 14.86±2.5 | 0.64 |
| Creatinine (mg/dl) | 0.56±0.05 | 0.60±0.02 | 0.58±0.007 | 0.61±0.01 | 0.32 |
* TC: Total cholesterol; TG: triglycerides; HDL-C: high-density lipoprotein-cholesterol; LDL-C: low-density lipoprotein-cholesterol; VLDL-C: very low-density lipoprotein-cholesterol; ALT: alanine aminotransferase; AST: aspartate aminotransferase. abc Mean among the same row carrying different superscripts are markedly different at (P ≤ 0.05).
Table 4: The influence of dietary PFA inclusion on economic parameters of broiler chicks (means± SEM).
| Trait studied | Experimental diets | P-value | |||
| Control | Phytogenic feed additive (PFA) | ||||
| 0.05% | 0.10% | 0.20% | |||
| Feed costs $/chick |
1.69±0.007c |
1.731±0.004b |
1.71±0.005b |
1.75±0.003a |
0.001 |
| Total expenses $/chick |
2.63±0.007 c |
2.67±0.004b |
2.65±0.005b |
2.69±0.003a |
0.001 |
| Total return $/chick |
3.36 ±0.01 c |
3.35±0.01c |
3.63±0.01b |
3.78 ± 0.01a |
0.001 |
| Gross margin $/chick |
0.73± 0.02c |
0.68±0.01c |
0.98± 0.01 b |
1.09± 0.10a |
0.001 |
| Benefit cost ratio |
0.22±0.00c |
0.20±0.002d |
0.27±0.003b |
0.29±0.003a |
0.001 |
| Economic efficiency% |
43.19±1.34c |
39.28±0.60 c |
57.31±1.19b |
62.28±0.98a |
0.001 |
| Feed cost $/ kg gain |
.769 0±0.02b |
0.787±0.02a |
0.720±0.06c |
0.704±0.02c |
0.001 |
abc Mean among the same row carrying different superscripts are markedly different at (P ≤ 0.05). Cost/kg diet$ =control (0.381), PFA 0.05% feed= (0.388), PFA 0.10% feed= (0.394), PFA 0.20% feed= (0.407). Price of meat = $ 1.5 and fixed costs = $ 0.937.
Antioxidant enzymes
Figure 1 presents the effects of the PFA on broiler relative mRNA gene expression profile of anti-oxidant enzymes (GST/GAPDH, SOD/GAPDH, and Catalase/GAPDH). The highest relative mRNA of GST/GAPDH (P<0.05) were found in the broilers fed on a diet containing 0.20% PFA followed by those fed on a diet containing 0.10 and 0.05% PFA compared to un-supplemented groups. In the same trend, the relative mRNA of SOD/GAPDH was improved (P<0.05) in groups fed on a diet containing 0.20 and 0.10% PFA followed by those fed on a diet containing 0.05% PFA if compared with the control group. While, the highest relative mRNA of Catalase/GAPDH (P<0.05) were found in the birds fed on a diet containing 0.20% PFA compared to other treatment groups.
Due to a large number of various phytogenic formulations with different phytogenic compounds existing in the market, facts of scientific trials cannot be compared definitely. Many trials have reported the beneficial impacts of PFA as growth promoters when used in poultry feed (Orayaga et al., 2016; Aguihe et al., 2017). In our research, the inclusion of PFA containing diets had a strong effect on the growth performance of the broiler. These results agreed with some reports showing that the broiler performance was positively affected by dietary inclusion of PFA (Abdel-Hafeez et al., 2015; El-Ashram and Hamady, 2020). This beneficial effect of PFA supplementation in a dose-dependent manner on growth performance could be referred to its high concentration of biologically active substances that have a vast extent of action, like anti-oxidant, anti-bacterial, hypolipidemic, digestive stimulant, growth promoter, immunomodulator, anti-toxigenic, anti-parasitic, anti-viral, insecticidal, and anti-mycotic action (Marappan et al., 2014). These properties of PFA lead to lower the pathogenic microbes, and safeguard the gut microvilli (Hashemipour et al., 2013; Ksouri et al., 2017); improving appetite, the release of digestive enzymes, nutrient digestibility, and metabolic processes resulting in the enhancement in the concentrations of hepatic gluconeogenic enzymes (Sheikh et al., 2015) or liberate antibiotic-like bacteriostatic and/or bactericidal impacts on different pathogenic gut micro-organisms (Mueller et al., 2012).
Biochemical blood parameters of the broilers are usually related to their health condition. These parameters represent a valid index of both the nutritional and physiological conditions of broilers. In our study, plasma creatinine concentration didn’t vary between the treatments which exhibit the non-pathological and non-toxic impact of PFA on the kidney. ALT and AST are intracellular enzymes accumulating in the heart, and liver, as well as their enhanced action, is the signal of heart and liver damage (Nyblom et al., 2006).
In our trial, there was no marked change in the serum TAG, HDL-C, VLDL-C, ALT, AST, urea, and creatinine activities among broilers fed PFA containing diets. Serum ALT and AST are considered a useful parameter to determine a safe addition level for new additives and non-commonly ingredients (Diaz et al., 2003). Similarly, activities of ALT and AST in serum among broilers fed diets containing PFA were among the normal extent, which concluded that birds fed 0.00, 150, and 300 ppm diet wasn’t toxic to the birds (El-Ashram and Hamady, 2020). Also, no changes were recorded in the mean values of ALT and creatinine among the treatment groups fed on Hippophae rhamnoides extract as PFA (Kalia et al., 2018). In contrast to our results, Reis et al. (2018) has recorded that serum levels of ALT and AST were reduced in birds fed with either concentration (0.05 and 1%) of PFA on day 14 of life, and the same was noted concerning AST in birds supplemented with 0.50% of the PFA. Furthermore, Kalia et al. (2018) mentioned that a marked higher level of HDL, AST level was lower and no changes were recorded in plasma TAG level among the treatment groups were observed in the experimental group. This could have been due to the hepatoprotective efficiency of PFA in the broiler’s liver cells (Maheshwari et al., 2011).
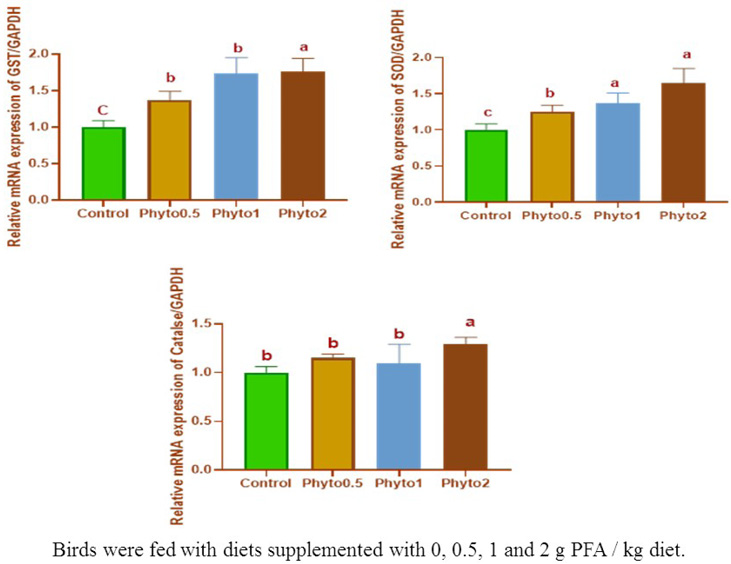
Figure 1: The influence of dietary PFA inclusion on relative mRNA gene expression profile of anti-oxidant enzymes (GST/GAPDH, SOD/GAPDH, and Catalase/GAPDH) of broiler chicks.
The main role of the LDL is to transport the TC to peripheral tissues from the liver and it referred to as bad cholesterol. In our work, the TC and LDL-C showed a marked reduction in the broiler group fed on a diet containing 0.20% PFA when compared with other groups. On the similar ground of our results, mean values of plasma TC and LDL-C were markedly lower in treatment group broilers as compared to the control group at 3 and 6 weeks (Kalia et al., 2018). Reduced level of LDL-C in treatment group birds might be due to the capability of polyphenolic substances to reduce apolipoprotein A-1(apoA-1) and apolipoprotein B (apoB) secretion (Teriault et al., 2000). Reduced levels of TC might be due to the inhibitory impact of flavonoids on the efficiency of acetyl coenzyme acetyl-transferase and 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) reductase, key regulatory enzymes in TC biosynthesis (Lien et al., 2008).
From an economic point of view, feed is a major input for the broiler rearing costs which contributes approximately 70-80% of the total production cost. The results showed that increasing feed cost and total expenses with increased PFA level in broiler diet was attributed to the additional cost from using PFA in the diet. Improvement in the economic values: total return, net profit, feed costs/kg weight gain) and the subsequent improvement of benefit/cost ratio and economic efficiency resulted from adding PFA in broiler diets which led to better feed utilization, increased weight gain, and better feed conversion ratio, which ultimately led to higher return (Tanzim et al., 2017). The extra costs of adding PFA to the broilers diet was negligible, these results agreed with the previous report of Karangiya et al. (2016) who reported that adding herbal additives to bird diets increased production costs of broilers. Also, Puvaca et al. (2016) suggested that the lowest feed cost/kg body weight gain can be obtained using herbal powder supplementation in the broiler diets. Moreover, using commercial herbal preparation in broiler diets improved economic efficiency. This improvement could be due to improving the FCR or lowering the quantity of feed that wanted to make one unit of meat Omar et al. (2016) and Singh et al. (2018).
Similarly, to our expectations and to data from Lii et al. (2010), PFA has generally increased the mRNA expression of anti-oxidant genes in the liver. This up-regulation effect may be due to the anti-oxidant safeguard accomplished by the inducement of these genes in the liver and protected the organism against oxidative stress which found a special up-regulation of GST- and SOD enzyme activity by PFAs compared to the control (Mueller et al., 2012). In contrast to our data, feeding diets supplemented with PFA to rats and mice regarding a down-regulation of liver antioxidant enzymes (Schrader et al., 2011; Mueller et al., 2012). Many trials have elucidated that the lack of GST- and SOD enzymes support the growth of chronic inflammatory gut diseases. Furthermore, it has been displayed that GST hinders the aquaporin-mediated absorption of peroxides into the organism and impedes the up-regulation of the pro-inflammatory inducible cyclo-oxygenase (Esworthy et al., 2011) or the increased gut barrier against inflammatory and pro-oxidants stress saves peripheral organs such as the liver from oxidative stress are further promoted by GST- and SOD (Mueller et al., 2012). The higher anti-oxidant enzymes values of all chickens receiving a PFA, propose that the livers of supplemented birds had a lowered oxidative stress and raised anti-oxidative capacity, making the further inducement of anti-oxidant enzymes unnecessary (Schrader et al., 2011).
CONCLUSIONS AND RECOMMENDATIONS
Dietary supplementation with PFA in broiler chicken could provide a safe growth enhancing effect without any negative outcome on biochemical parameters. Moreover, PFA rich broiler diets could progress economic efficiency through maximize return and gross margin. The up-regulation of relative mRNA expression of anti-oxidant genes in the liver may demonstrate a new and attractive mechanism by which PFA promotes the general health of broilers. Finally, keeping in view the eco-friendly nature and beneficial effects of PFA, it can effectively use as an alternative to replace the antibiotic growth promoters in the poultry industry.
ACKNOWLEDGEMENT
The authors would like to express their heartfelt appreciation to the staff of the Faculty of Veterinary Medicine, Zagazig University, Egypt to support in the accomplishment of this research.
AUTHOR’S CONTRIBUTION
All authors contributed equally to this work.
CONFLICTS OF INTEREST
The authors have declared no conflict of interest.
REFERENCES





