Journal of Animal Health and Production
Review Article
How to Alleviate Side Effects of Anti-Neoplastic Drug Cisplatin by using Nanotechnology?
Sawsan Mohamed El-Sheikh, Nagah Edrees, Hend El-Sayed*, Naglaa Zakria Eleiwa
Department of Pharmacology, Faculty of Veterinary Medicine, Zagazig University, Zagazig 44511, Egypt.
Abstract | Cisplatin is a potent, first-line chemotherapeutic drug used for a variety of solid tumors in pet animals as well as humans. The therapeutic application of cisplatin is restricted due to its resistance and toxicity to the healthy tissues. In addition, it exhibits less selectivity for tumor against normal tissue that may be a crucial limiting element for its worth. To handle these limitations of the free drug, nanotechnology-based therapeutics have shown clear benefits compared to unmodified drugs, including better half-life, survival, performance targeting and less side effects for patients. Nanoparticle drug delivery systems have been investigated to facilitate the delivery of cisplatin to kill cancer cells. Nanoparticles/drug conjugate design has important effects on pharmacokinetics, bio-distribution of the drug which reduces its observed toxicity. Formulations that allow enhanced delivery and controlled release of anti-tumor drug can actually accomplish the goal of nanomedicine as a means of enhancing effectiveness and minimal serious side effects. Here, we survey some of the most common adverse effects associated with cisplatin conventional therapy with highlights latest progress in formulations of nanoparticles; concentrating on the promising agents in preclinical or clinical studies; as smart nanocarriers conjugate that composed of metal nanoparticles as a delivery vehicle for cisplatin to target the tumor mass and reduce its side effects and tissue toxicity.
Keywords | Cisplatin, Drug Conjugate, Nanotechnology, Side effects, Silver nanoparticles
Editor | Asghar Ali Kamboh, Sindh Agriculture University, Tandojam, Pakistan.
Received | November 05, 2020; Accepted | November 15, 2020; Published | December 27, 2020
*Correspondence | Hend EL-Sayed, Department of Pharmacology, Faculty of Veterinary Medicine, Zagazig University, Zagazig 44511, Egypt; Email: Henda.shaheen@gmail.com
Citation | El-Sheikh SM, Edrees N, EL-Sayed H, Eleiwa NZ (2020). How to alleviate side effects of anti-neoplastic drug cisplatin by using nanotechnology? J. Anim. Health Prod. 9(s1): 1-8.
DOI | http://dx.doi.org/10.17582/journal.jahp/2020/9.s1.1.8
ISSN | 2308-2801
Copyright © 2020 El-Sheikh et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Cisplatin is a well recognized and efficient chemotherapeutic drug used to treat many human and animal cancers including cancers of the bladder, head and neck, lung, ovarian and testis. It is useful for treating a wide range of tumor forms including carcinomas, germ cells tumors, sarcomas, and lymphomas in cats and dogs (Tozon et al., 2001). Encouraging animal experiments led to the introduction of cisplatin to laboratory studies in 1971, and in 1978, was the first platinum compound approved by the FDA for cancer treatment (Kelland, 2007).
While patients adjust to the preliminary cisplatin therapy, resistance usually emerges through a variety of possible pathways (Galluzzi et al., 2012). Resistance to cisplatin may be either attained during ongoing drug therapy or may be a feature of an intrinsic tumor (Rabik and Dolan, 2007). The maximum doses of cisplatin needed to combat resistance can result in severe toxic effects as myelotoxicity, neurotoxicity, cardiotoxicity and nephrotoxicity because cisplatin destroys malignant and healthy cells the same as all other chemotherapeutic agents (Borch and Markman, 1989).
Mechanism of action of cisplatin
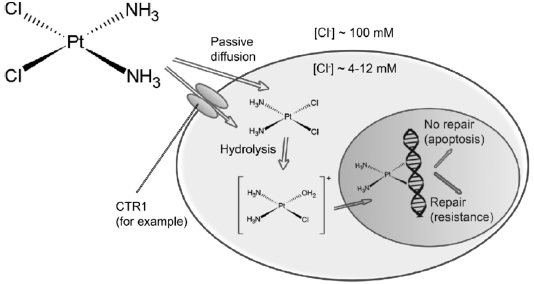
Following intravenous route, the drug flows from blood to cells via both passive diffusion and active transmission (Gately and Howell, 1993). Cisplatin was meant to reach cells solely by passive diffusion, but the data collected over the last few years suggested active mechanisms (Filipski et al., 2008).
Before the drug can react with its target, sequential aquation reactions must activate the neutral cisplatin molecule, which substitutes the cisplatin chloro ligands with water molecules (Hincal et al., 1979). The significant decrease in the concentration of chloride in the cytoplasm relative to the blood makes cisplatin easier to acquire (Davies et al, 2000). This form of the drug is very reactive and can be down regulated intracellular by interacting with several thiol-containing nucleophiles, such as glutathione (Kelland, 1993). Cisplatin is thought to do its cytotoxic action after aquation by combining to DNA bases and developing adducts that ends up causing cell death (Figure 1) (Pinto and Lippard, 1985).
Cisplatin causes cytotoxicity by binding to genomic DNA (gDNA) and non-DNA targets (GSH, MT) and causes necrosis and apoptosis in heterogeneous tumor mass cell populations (Fuertes et al., 2003). Cisplatin-formed DNA adducts are known to interfere with DNA replication (Sorenson and Eastman, 1988). This leads to distortion in the DNA structure (Takakura and Hashida, 1996). These structural DNA distortions are recognized and coupled by several key pathways, which cause different signaling series that can result in cell death (Chaney et al., 2004).
Cisplatin also increases oxidative damage by boosting reactive oxygen and reactive nitrogen species production causing injury to subcellular structures and macromolecules like DNA, lipids and proteins (Dos Santos et al., 2012).
Cisplatin resistance
Initial response of cancer cells to cisplatin-based chemotherapy is generally high; however, there is a delayed retrogression due to the production of cisplatin resistance (Yang et al., 2012). Resistance to cisplatin is regulated through two separate processes: In the first scenario, binding of platinum DNA may be insufficient which arise from reduced drug absorption, elevated drug evasion or high levels of intracellular thiol-containing agents, like glutathione, attach to the platinum core causing its inactivation (Holzer et al., 2006).
The second mechanism may be mediated through resistance after DNA binding owing to exclusion of DNA adducts or increased susceptibility to adducts in tumors with hyperactive nucleotide-excision repair NER (the key repair pathway for platinum-DNA adduct removal) because of factors including increased activity of the DNA excision repair endonuclease protein ERCC1 (Dabholkar et al., 1992).
Cisplatin side effects
Using maximum doses of cisplatin to overcome resistance can result in severe tissue damage, as cisplatin, is non-selective and has a cytotoxic effect on both healthy normal and malignant cells (Oun et al., 2018).
Although successful against tumors, cisplatin therapy has serious side-effects such as nephrotoxicity. Clinical symptoms of acute and/or chronic nephrotoxicity include decreased renal plasma production, glomerular filtration rate, increased serum creatinine, and decreased serum magnesium and potassium levels (Pabla and Dong, 2008).
Studies in experimental animals demonstrating that the kidneys appear to pile up more cisplatin than other organs, and that the proximal tubules are the renal structure directly impaired by cisplatin. Nephrotoxicity can be explained partly because the kidney retains more cisplatin than any other organ also it is the key excretory organ for the administered drug. Cisplatin mainly absorbed by the renal tubules and the apoptosis tissue damage caused by it may trigger extreme and possibly irreversible renal failure (Miller et al., 2010).
Cisplatin is concentrated in the renal proximal tubule cells causing nephrotoxicity, featured by cellular damage, loss of microvillus, mitochondrial vacuolization and functional modifications involving: blockage of protein synthesis, reduction of the activity of antioxidant enzymes by reduction of glutathione, lipid peroxidase, and organelle dysfunction (Satoh et al., 2003). Cisplatin was able to develop active oxygen species (ROS) such as superoxide anions and hydroxyl radicals and to inhibit antioxidant enzyme activity in the renal tissue (Hennessy et al., 2002).
Nephrotoxicity involved changes in electrolytes (hyperK+, hypoK+, hyperCa++, hypoNa+, hypoCl+), increased blood nitrogen, urea and creatinine, hematuria, polyuria, oliguria, urinary tract infections, and renal colic to renal insufficiency (Astolfi et al., 2013).
Most patients treated with cisplatin experience symptomatic and clinically observable sensory neuropathy due to their preferential absorption in the dorsal root ganglia (DRG), which results in broad sensory fibers neuropathy. Neurotoxicity affected primarily the peripheral system compared to the central nervous system. Paresthesia accompanied by headache, speech disorder, dysphasia, encephalopathy, syncope, seizures, panic and transient ischemic attacks, vision loss, repetitive strain injury and lack of motor control were the most common symptoms (Albers et al., 2011).
Ototoxicity is another significant complication in up to 80 percent of patients received cisplatin, described as damage to the auditory nerve or to the vestibular system of the ear. This is triggered by the death of mechano-sensory hair cells in both the outer and inner ears that turn physical stimuli into neuronal impulses that enable organisms to hear and at least 75 percent of human deafness is triggered by their death (Cardinaal et al., 2000).
Astolfi et al. (2013) analyzed data from 123 patients with different tumor types undergoing treatment revealed adverse effects of cisplatin, including myelosuppression causing blood disorders such as deficiency of red blood cells, white blood cells, neutrophils and thrombocytes.
Cardiotoxicity was also related to treatment with cisplatin. The cardiotoxicity results in lactate dehydrogenase (LDH) leakages as well as creatine kinase (CK) from the cardiac myocytes. This could be secondary processes resulting from cardiac membrane lipid peroxidation that is induced by cisplatin (Akman et al., 2015).
While cisplatin-induced kidney injury has attracted significant interest, the cardiotoxicity triggered by cisplatin remains elusive (Topal et al., 2018). Other effects, such as acute and chronic cardiovascular problems, have also been identified which may affect the quality of life of the patient. Electrocardiographic alterations, myocarditis and cardiomyopathy are known to be significant clinical symptoms. These cardiac shifts, which lead to reduced total dose of cisplatin, may also involve stopping of chemotherapy (Varga et al., 2015).
Oxidative stress, apoptosis, and inflammation are generally associated with cisplatin-induced cardiac injury (Dugbartey et al., 2016). Cisplatin typically causes mitochondrial dysfunction and lowers antioxidants in cancer patients’ tissues during cisplatin treatment, leading to ROS excessive production and resulting cellular degeneration. Consequentially, the overloaded oxidative damage triggers tissue changes after multiple cisplatin doses, such as fibrosis and oedema. In addition, excessive development of ROS can generate inflammatory reactions through activation of the NF-πB signal pathway; this contributes to enhanced pro inflammatory cytokine release in cisplatin-induced diseases (Mukhopadhyay et al., 2011).
Bcl-2 (an apoptosis regulator) plays a vital role in the apoptosis process while Bax is a significant regulator of Bcl-2 function (Zhu et al., 2017). When cisplatin stimulates ROS, Bax is transferred to the outer membrane of the mitochondria, and changes its permeability; this opens the mitochondrial pores and trigger cytochrome C leakage into the cytosol, this activates the pro-apoptotic caspase 9 and its caspase-dependent downstream sequence (Marullo et al., 2013).
Cisplatin caused cardiac and hepatic injuries that were indicated by elevation of serum hepatic and cardiac injury markers as well as proinflammatory cytokines. Moreover, it decreases in the activities of antioxidant enzymes, a decrease in glutathione concentration, and an increase in malondialdehyde level. Cisplatin also resulted in histopathological myocardial and hepatocellular changes, and overexpression of p53 and COX-2 in cardiac and hepatic tissues (Abdellatief et al., 2017).
Nanotechnology based drug delivery
The aforementioned side effects make it impossible for a large number of patients to get the full benefit of the treatment. Beside the adverse effects, the body also experiences a loss of drug activity linked to inadequate circulation and delivery of the drug to the tumor cells, along with deactivation processes that completely change the chemistry of these molecules before reaching the cancer cells (Reedijk, 2003). To overcome these difficulties, a wide variety of drug carriers of nanoparticles (NP) have been investigated as anticancer drug delivery systems to facilitate maximum accumulation of the drug in tumor tissue and to further minimize harmful effects (Duan et al., 2016).
The concept of a “Magic Bullet” proposed by a German physicist nicknamed (father of chemotherapy) was reported; a compound that could be developed for specifically killing diseased cells (Strebhardt and Ullrich, 2008). If this compound could be used to specifically administer anticancer drugs straight to tumors, there would be no need to think more about off-goal side effects, since the medication will only be eligible for action at the intended site. A lot of work has been conducted to create a device that can precisely guide drug delivery to cancer cells, and success in this area is accelerating. Drug delivery systems depending on nanoparticles have made dramatic changes to the controlled drug release, in particular, anticancer drugs due to their physiochemical properties (Lim et al., 2013).
Nanotechnology described as the comprehension and control of matter at dimensions between 1 and 100 nm where specific phenomena allow for novel concept (Hulla et al., 2015). Compared with unmodified drugs, nanotechnology-based therapies showed significant benefits, including improved half-lives, survival, performance targeting and fewer side effects for patients (Gandhali, 2016).
In order to ensure successful delivery of drugs, two main concepts should be involved, first, that the drug should be able to enter the target tumor site after injection with minimal loss of volume and function in the circulation of the blood. Second, drugs can target only tumor cells without causing harm to normal tissue (Rajshri and Tarala, 2007). Combined anticancer drug and nanoparticle therapy facilitates synergy and suppresses drug resistance by distinct low-dose modes of action. Nanoparticle-combined drug therapy increases the efficacy of an antitumor drug, eliminates possible adverse effects and improves its bioavailability (Hu et al., 2010). Previous research developed and synthesized Nano drug co-delivery systems with various anti-tumor drugs. However, medications displayed more effectiveness in the nano-drug co-delivery system than free drugs due to the disparity in drug release rates (Jain et al., 2010; Wang et al., 2011; Feng et al., 2014).
Classification of nanomaterials
As classified by Jeevanadam et al. (2018), most of the existing NPs can be divided into four material-based categories:
Methods of nanoparticles synthesis
As far as the production of nanoparticles is concerned; which may be natural or synthetic in origin; has specific characteristics at the nanoscale. Basically two techniques, including a variety of preparation methods, are applied. The first technique is the “top-down” that requires the application of external force to decompose solid materials into small pieces. In this technique various physical, chemical and thermal tools are used to create the energy essential for the production of nanoparticles (Iravani, 2011). The second technique, regarded as “bottom-up,” depends on collecting and merging atoms or molecules in gas or liquid. These two techniques possess respective advantages and drawbacks. In the up-down approach, which is more expensive, perfect surfaces and edges cannot be obtained owing to the cavities and unevenness which might occur in nanoparticles; while other amazing results of nanoparticles synthesis can be achieved from the bottom up method. Furthermore, in the bottom-up approach, no waste materials that need to be discarded and better control of the size of the nanoparticles can be acquired (Makarov et al., 2014).
Various methods of metal nanoparticles synthesis lead to varying sizes, forms, morphology and also stability (Dhand et al., 2015). Generally, such approaches can be listed as:
Natural “Green” synthesis method
The biological (green) method; described as an alternate to chemical and physical processes; offers an ecologically safe route to synthesize nanoparticles. Furthermore, this approach doesn’t need costly, dangerous or toxic chemicals. Thanks to the biological approach, metal nanoparticles with different characteristics can be successfully prepared in recent years. In one step, the synthesis can be achieved using natural organisms such as bacteria, yeasts, molds, algae and plants or their products. Nanoparticles are synthesized by reduction using molecules in plant and micro-organisms including enzymes, proteins, amines, phenol compounds, pigments and alkaloids (Shah et al., 2015).
In conventional methods, reducing agents responsible for the reduction of metal ions and stabilizing agents used to prevent excessive aggregation of the resulting nanoparticles pose a possibility of atmospheric and cell-toxicity. In addition, the contents of the formed nanoparticles are known to be toxic in terms of shape, size and chemical structure. Nevertheless, nanoparticles with bioactivity are produced in the green synthesis process, and the reducing agents are naturally present in the biological entities used, making them the safest process (Hussain et al., 2016).
Nano-carrier properties
Nanocarriers’ properties, including their sizes, high surface to volume ratios, controlled drug release patterns, and targeted modifications, enable them to better reach target tumor tissue and release drugs in a safe, regulated manner (Wicki et al., 2015). Size is critical for moving through the bloodstream and for delivering the nanocarriers to tumor tissue afterwards. Although smaller nanoparticles accumulate more easily in leaky blood vessels of tumors than larger ones, they may also penetrate into normal tissue. On the other hand, the extravasations of larger nanoparticles are not as simple and therefore their distribution in the bloodstream is unpredictable (Bregoli et al, 2016). Nanoparticles with hydrodynamic diameters between 30–200 nm can penetrate easily through the leaky vasculature of tumours and persist in tumours for long periods, generally referred to as enhanced permeation and retention impact (EPR) (Dogra et al., 2018).
Fluid dynamics can be influenced by the shape of the nanocarriers and thus influence uptake. At present, the use of spherical ones appears to be more widespread (Truong et al., 2015).
The charge of nanocarriers can also have an effect on their blood stability and distribution. Positively charged nanoparticles have previously been shown to target tumor vessels quite efficiently, but a shift to a neutral charge has allowed the nanoparticles to spread more rapidly to the tumor tissue (Stylianopoulos et al., 2010).
Surface modifications of the nanoparticles, such as ligands to over-expressed receptors, can help to precisely absorb the drugs in the tumor tissue. Regulated release mechanisms can also prevent the toxic drug from being administered unspecifically to normal tissue (Locatelli and Franchini, 2012).
Cisplatin and nanoparticles conjugates, preclinical studies
Gounden and Singh (2019) explored silver nanoparticles (AgNPs) as a way to deliver therapeutic material to the nucleus, thereby attacking diseased cells. After encapsulation of Cisplatin using a silver NP chitosan (CS) biopolymer, the cytotoxicity profiles of the CS-AgNP-CIS nano-complexes using sulforhodamine (SRB) and MTT assays revealed significant cell death in the various breast cancer cell lines. The Nano complexes were evidently more effective than the free drug, displaying more than 50% cell death at lower concentrations.
A study by Ramezania et al. (2019) intended to restore sensitivity to cisplatin to A2780 cisplatin-resistance cell lines in the presence of naturally synthesized curcumin-coated silver nanoparticles (cAgNPs). Synergic cellular effects of cAgNPs and cisplatin on cisplatin-resistant ovarian carcinoma 2780 were evaluated using MTT assay. Cell death induction in the combined group in A2780 cells increased significantly, compared to the free cisplatin or cAgNPs, according to the findings.
On the same ground, conjugation of cisplatin and gold nanoparticles by a thiolated oligonucleotide linker (CIS-AuNPs) exhibited superior potency on the A549 (adenocarcinomic human alveolar) cell line with an IC50 value of 0.9 μM versus 11 μM for cisplatin alone (Dhar et al., 2009).
In a recent research was conducted to assess the therapeutic effectiveness of the cisplatin embedded in polybutylcyanoacrylate (PBCA) nanoparticles for the treatment of renal cancer. Cisplatin/ loaded PBCA increased the cytotoxicity effects of cisplatin against renal cell adenocarcinoma cells (2.3-fold) and substantially reduced the concentrations of blood urea nitrogen (1.6 fold) and creatinine (1.5 fold) relative to free cisplatin. This nanocomplex also induced a 1.8 fold improvement in the therapeutic effects of cisplatin, where a decrease in the mean tumour size (3.5 mm versus 6.5 mm) was observed relative to cisplatin treated rats (Ghaferi et al., 2020).
A study also recently formulated cisplatin/ loaded biodegradable nanoparticles for the treatment of epithelial ovarian cancer (EOC). Cell cytotoxicity was assessed following exposure of cisplatin or cisplatin-NP conjugate to the cell culture. The efficacy of intraperitoneal chemotherapy was also tested in a xenograft model of SKOV3-Luc cells in mice. The findings revealed that the highest intracellular platinum concentration was achieved with cisplatin-NP conjugate and significantly improved efficacy. Aligned with in vitro findings, cisplatin-NP conjugate has been shown to largely reduce tumor volume in vivo compared to free cisplatin. The researchers assumed that nanoparticles encapsulated cisplatin serve as an intracellular repository that increases cisplatin performance, along with an extended release profile (Bortot et al., 2020).
CONCLUSIONS AND RECOMMENDATIONS
The formulations of nanoparticles have been developed to improve the delivery of drugs and have the ability to promote the selective accumulation of cisplatin in tumor cells without increasing off-target impacts, toxicity as well as reducing its side effects. Cisplatin/ nanopaticles conjugate are a promising candidate that can boost efficacy and reduce the side effects and toxicity caused by free cisplatin, according to the preclinical studies. Further studies are required concerning the mechanism, safety and therapeutic range of application of such conjugates.
Author’s Contribution
SME writing review and supervision. NE data curation and supervision. NZE conceptualization, writing review, editing, visualization, supervision. HE investigation, resources, writing original, editing.
Conflict of interest
The authors have declared no conflict of interest.
REFERENCES