Journal of Animal Health and Production
Research Article
Determination of the Effect of Ecthyma Vaccine Virus on Immunity Against the Foot and Mouth Disease (Fmd) Vaccine in Sheep
Veli Gülyaz1*, Fahriye Saraç2, Can Çokçalışkan3, Esra Satır2, Beyhan Sareyyüpoğlu3, Abdullah Arslan3, Serdar Uzar2, Mehmet Karakaya3, Osman Kara3, Gonca Öztap1
1Ministry of Agriculture and Forestry, General Directorate of Food and Control Çankaya, Ankara, Turkey; 2Pendik Veterinary Control Institute, Pendik, Istanbul, Turkey; 3Institute of Foot and Mouth Disease (SAP), Çankaya, Ankara, Turkey.
Abstract | Foot and Mouth Disease (FMD) is highly contagious viral disease of cloven-hoofed animals with huge economic losses because of significant decrease of yield. One of the most basic methods in the fight against FMD is vaccination in many countries. Trivalent or tetravalent inactivated FMD vaccines are used in the fight against FMD extensively. Although, FMD vaccine potency mainly depends on FMD antigen amounts in the vaccine composition as micrograms, it has been required to increase immunity against inactivated FMD vaccines with some immunomodulators or immunostimulants like some bacterial or viral antigens. Therefore, this project was designed to investigate the immune-stimulating effect of ecthyma virus in sheep when it was applied together with FMD vaccine either in same formulation or simultaneous injections at different sites of the body. Level of immunity was measured by virus neutralization test (VNT) and liquid phase blocking ELISA (LPBE). According to our results, the highest antibody titers of virus neutralization (VN) and LPB-ELISA were obtained by single administration of FMD vaccine, followed by simultaneous and combined administrations respectively at both day 30 and 60. However, this difference was statistically non significant (p < 0.05). Thus, it could be concluded that the use of ecthyma virus as an immune stimulant did not cause an increase in antibody level against FMD virus.
Keywords | Foot and mouth disease, ecthyma, Immunity, Vaccine
Received | September 20, 2019; Accepted | November 02, 2019; Published | February 19, 2020
*Correspondence | Veli Gulyaz, Ministry of Agriculture and Forestry, General Directorate of Food and Control Çankaya, Ankara, Turkey; Email: veli.gulyaz@tarimorman.gov.tr; veligulyaz@yahoo.co.uk
Citation | Abousenna MS, Amal AM, Aziz HA, Barghooth WM, Shafik NG (2020). Determination of the effect of ecthyma vaccine virus on immunity against the foot and mouth disease (fmd) vaccine in sheep. J. Anim. Health Prod. 8(1): 19-26.
DOI | http://dx.doi.org/10.17582/journal.jahp/2020/8.1.19.26
ISSN | 2308-2801
Copyright © 2019 Gulyaz et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Foot and mouth disease (FMD) is caused by a virus of the genus Aphthovirus, family Picornaviridae. There are seven serotypes of FMD virus (FMDV), namely O, A, C, SAT1, SAT2, SAT3, and Asia 1 that infect cloven-hoofed animals. The virus has a diameter of 26 nm and contains a nonenveloped single-stranded ribonucleic acid (RNA) genome and has icosahedral symmetry. It has a protein-structured capsule and a positive polarity of approximately 8.4 kb (OIE 2018). Infection with any one serotype does not confer immunity against another. Many strains can be identified by biochemical and immunological tests within serotypes. The signs can range from a mild or inapparent infection to one that is severe. Death may result in some cases especially in calves. Mortality from a multifocal myocarditis is most commonly seen in young animals, myositis may also occur in other sites. Routine vaccination against FMD is used in many endemic countries or the disease free areas in which the absence of infection was provided by vaccination (San Segundo et al., 2017). In contrast, a number of disease-free countries have never vaccinated their livestock, but have preferred the use of strict movement controls and culling of infected and contact animals when outbreaks have occurred. The vaccines are formulated for their specific purpose both aluminium hydroxide, saponin and oil adjuvant vaccines have been used. The immunogenicity of FMD vaccines varies according to the amount of micrograms of the virus antigen and types introduced into the vaccines, but the effects of the adjuvant types are also important. Moreover, the addition of immunostimulatory antigenic proteins into the vaccines causes a high humoral and cellular immune response to the vaccine used. Vaccines with 6 and 10PD50 (50% protective dose) protection values in sheep, goats and cattle are used to obtain immunity against FMD by many countries such as in Asia, Middle East, Africa, including Turkey (Hussain et al., 2017; Khorasani et al., 2016; Lyons et al., 2017; Sareyyüpoğlu et al., 2019).
Contagious ecthyma (CE) is a zoonotic infectious disease primarily of domestic sheep and goats which is caused by contagious ecthyma virus (CEV) which has a close morphological, immunological and genomic relationship with the Parapoxviruses of cattle (Chan et al. 2017; Zeedan et al. 2015). The disease is commonly termed as ORF, contagious pustular dermatitis, scabby mouth and sore mouth (McKeever et al., 1988; Robinson ve Balassu, 1981). In flocks that have never had soremouth, nearly all exposed animals will develop the disease. The virus is transmitted to susceptible animals and human via the direct contact (Demiraslan et al, 2017; Nashirudddullah et al., 2016). The virus penetrates through small abrasions in the skin. Even very minor damage to the skin may allow the virus to enter. Abrasions caused by forage are usually adequate for infection to occur. Carrier or chronically-infected animals may also serve as reservoirs for infection (Al Saad et al., 2017; Rafii and Burger, 1985).
It has long been known that an inactive ORF virus strain displays immunomodulatory properties in various animal species (Weber et al., 2007). There are even commercial preparations (such as Baypamun-Bayer) made with this agent and used in animal health for many years. The agents included in the Baypamun patent are avipox virus HP-1, parapox virus, ORF D1 701, vaccinia virus MVA, canarypox virus KP-1. It is possible to use these agents for therapy of various diseases including infections and cancers, and as an adjuvant in a wide range of areas (Hirth-Dietrich et al., 2003). Similarly, in this study, we tried to increase the level of immunity against the FMD vaccine by using immunomodulatory feature of inactive ecthyma virus. For this purpose, trial was conducted to clarify simultaneous and combined vaccination of FMD with ecthyma vaccine on immune response of sheep.
MATERIALS AND METHODS
Vaccines
Inactive ecthyma vaccine virus E(P)CK22 with a titer of at least TCID50 106.0/ml was obtained from Pendik Veterinary Control Institute, Turkey. Trivalent FMD vaccine containing A-TUR-14, O-TUR-14 and Asia1 (Asia-1 TUR-14) strains with 6PD50 was obtained from Ankara FMD Institute, Turkey.
The vaccine formulation of (FMD+ecthyma) was prepared at the Ankara FMD Institute. To form a double oil emulsion (WOW), after with sterile filtration using 0.45 and 0.2 µm cellulose acetate filter, adjuvant (Montanide ISA 206 BVG) and antigens were formulated in a volume of 54% and 46%, respectively. They were separately heated to 30°C to make the formulation. Once the antigen and adjuvant reached to the 30°C, the antigen was added into the adjuvant. The emulsion was monitored by adjusting the mixer speed, and the amount dispensed during this process. At the end of the dispensing process, rapid mixing was initiated to complete the emulsion. Then, the emulsion was completed and allowed to cool to 4°C. Sterility, harmlessness, pH, viscosity, conductivity, and drop tests were performed on the sample (Çokçalışkan et al., 2016; OIE, 2018).
Cell Culture
BHK-21-AN30 cell culture was used for VNT to determine antibody titers against FMDV in animals vaccinated with (FMD+ecthyma) vaccines.
Positive and Negative Control Sera
They were prepared against FMD virus types in Ankara FMD Institute Vaccine Control Laboratory, Turkey.
Combined Vaccine (FMD+ecthyma)
A total of 25 ml ecthyma virus suspension was mixed with 21 ml of inactive FMD O, A and Asia 1 virus strains at 8 µg (146S: 50 µg / ml) and 5 µg (146S: 50 µg / ml) and 4 µg (146S: 25 µg / ml) respectively in per dose of vaccine in sterile conditions. Then, 54 ml adjuvant (Montanide ISA 206 BVG/Seppic-Fransa) was sterilized through with 0,2 µ filter. To provide a stable emulsion (total volume of %54 adjuvant and %46 antigen), 54 ml oil adjuvant and 46 ml virus suspension (FMD+ecthyma) was warmed up to 30 ºC in an incubator with in separate containers. The virus suspension was added to the stirred oil adjuvant at 600-700 rpm as 5 ml /per min. in the laminar cabinet. The whole suspension of the virus was added to the adjuvant in the mixture, the speed of the stirrer was increased up to 1000-1200 rpm. The W / O / W vaccine emulsion obtained at the end of the process was divided in to the vaccine bottles and stored at +4 ºC until use.
Animals and Vaccination Route
The permission (Decision no: 16/2014) for animal use was obtained from the ethics committee of experimental animals of Pendik Veterinary Control Institute. A total of 35 FMDV-seronegative merino sheep aged 6-12 months were used. Sheep were divided into 4 groups (Table 1).
Group 1: Trivalent FMD vaccine was administered as 1 ml via intramuscular route to 10 sheep.
Group 2: Ten sheep were vaccinated by intramuscular route with 1 ml trivalent FMD vaccine at the neck area simultaneously by 1 ml subcutaneous route ecthyma vaccine at the anterior leg.
Group 3: One ml of the combined FMD+ecthyma vaccine suspension was injected to 10 sheep intramuscularly.
Group 4: As negative control, 1 ml PBS was were injected intramuscularly to the 5 sheep at the neck and subcutaneously to the 5 sheep. All vaccinated sheep were monitored for 30 days for body temperature, local temperature, lesions, appetite.
Table 1: Groups and number of animals
| Groups | Number of animals (n) |
| Group-1 FMD vaccine | 10 |
|
Group-2 (FMD+ ecthyma) vaccine simultaneously |
10 |
|
Group-3 (FMD + ecthyma) vaccines combined |
10 |
| Group-4 Negative control | 5 |
FMD: Foot and Mouth Disease
Collection of Samples
On the 30th days after vaccination, blood samples were collected and sera were stored at -20°C until tests were performed.
NSP (Non-structural Proteins) ELISA
The level of anti-NSP antibodies in animals were determined as recommended by the manufacturer of kit (Priocheck FMDV NS, the Netherlands) (Sørensen et al., 1998).
Virus Neutralization Test (VNT)
Antibody titer levels of FMD viruses were determined by VNT. Micro VNT was performed acording to the OIE, (2018). Glasgow-MEM containing 50 µl of fetal calf serum (FBS) were placed in all wells of a 96-well plate. 50 µl of the blood serum of each yearling was placed in the first four wells of the plate, and 50 µl of the first wells were transferred to the lower wells to make the two-fold dilutions of the serum. FMD virus strains in 50 µl of 100 TCID50 were added to two-fold dilution of the serum samples in the wells and let to neutralize for one hour at 37°C. At the end of the incubation period, 50 µl of BHK21 cell culture was added to all wells and incubated in 5% CO2 medium for 72 hours at 37°C. Cells were checked daily for CPE and evaluated by staining with crystal violet.
Liquid Phase Blocking ELISA (LPBE)
To determine immunity level of animals against FMD after vaccinations, LPBE was performed according to the method of Hamblin et al. (1986). Briefly, ELISA plates were coated with rabbit antibody (against anti-FMDV 146S antigens). Meanwhile, test and control sera were added to the carrier microplate. Then working dilution of FMDV type O, type A, and type Asia1 were added. The carrier and ELISA plates were incubated at 4°C. On the second day of the test, following washing of ELISA plate, mixture of serum/antigen was transferred from the carrier microplate to the ELISA microplate. Then, the plates were incubated at 37°C for 1 hour. After washing, 50 μl anti-FMDV type specific guinea pig antibodies were added and incubated at 37°C for 1 hour. Then 50 μl working dilution of the conjugate was added to the wells and incubated in a 37°C for 1 hour. Then, chromogen OPD/Substrate (H2O2) was added to each well, and incubated at room temperature for 15 minutes. Finally, 50 μl 1.25 M sulphuric acid was added to the wells. The absorbance was read by the microplate reader (Versamax, Moleculer Devices, USA) at 492 nm.
Statistical Analysis
All data were analyzed with Shapiro-Wilk and Levene Statistical tests. According to these two statistical results, Kruskal Wallis test was performed to detect the differences of antibody titers between the groups that vaccinated with single FMD, FMD+echtyma as simultaneously and FMD+echtyma combined on the 30th and 60th days. The statistical results were evaluated on the 95% confidence interval. SPSS 22.0 (Inc., Chicago II, USA) software was used.
RESULTS
VN Test
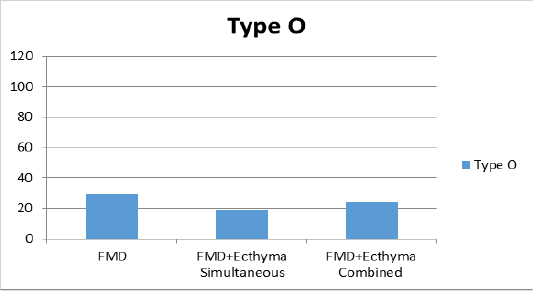
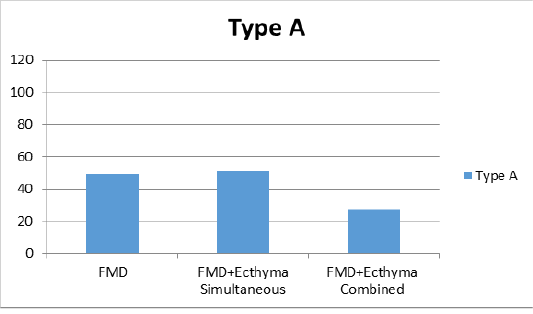
According to the average of VN test results in sheep after vaccination on the 30th day of study; the mean antibody titer values in all 3 groups against serotype O were found to be 1:29, 1:19 and 1:24 in groups 1, 2 and 3, respectively (Figure 1a). For the serotype A, only titer value was found to be 1:49 in the first group receiving FMD vaccine, while the mean titer was 1:51 in the second group where FMD and ecthyma vaccines were simultaneously administered; and 1:27 in the third group where both vaccines were combined (Figure 1b). The antibody titers against Asia1 serotype were found as 1:112, 1:69 and 1:50 in sera of vaccinated animals of all 3 groups respectively (Figure 1c).
LPBE Test
According to the mean LPBE test results in blood sera taken on the 30th day after the vaccination, the titer value of antibody levels against serotype O was found to be 1:99 only vaccinated with FMD, 1:63 in vaccinated with FMD and ecthyma vaccines simultaneously, and 1:14 in given
Table 2: The comparison of antibody titers differences between three groups vaccinated with ecthyma and FMD on 30th days.
| Antibody titers detection methods* | |||||||||||
| VNT | LPB ELISA | ||||||||||
| FMD Serotypes*** | Vaccinations | Animal Number (n) |
Mean Value |
Std. Deviation** |
Mean Rank | P value | Animal Number (n) |
Mean Value |
Std. Deviation |
Mean Rank | p value |
|
FMD O-TUR-14 |
FMD O-TUR-14 | 6 |
26.5 |
33.03 |
10.42 |
0.82 |
6 | 54.22 | 73.73 | 11.33 |
0.20
|
| FMD O-TUR-14 + Ecthyma simultaneously | 6 |
9.58 |
6 |
10.58 | |||||||
| FMD O-TUR-14+ Ecthyma combine | 6 | 8.50 |
6 |
6.58 |
|||||||
|
FMD A-TUR-14 |
FMD A-TUR-14 | 6 |
43.33 |
40.44 |
9.83 |
0.96 |
6 |
94.28 |
96.23 |
10.83 |
0.42 |
| FMD A-TUR-14 + Ecthyma simultaneously | 6 | 9.67 |
6 |
10.42 |
|||||||
| FMD A-TUR-14+ Ecthyma combine | 6 | 9.00 | 6 | 7.25 | |||||||
|
FMD Asia-1 TUR-14 |
FMD Asia -1 TUR-14 |
6 | 71.89 | 71.76 | 12.42 | 0.19 | 6 |
138.17 |
109.85 |
11.00 |
0.5 |
| FMD Asia -1TUR-14+ Ecthyma simultaneously | 6 | 9.17 |
6 |
9.92 |
|||||||
| FMD Asia-1 TUR-14+ Ecthyma combine | 6 | 6.92 | 6 | 7.58 | |||||||
*LPB ELISA: Liquid Phase Blocking-ELISA; VNT: Virus Neutralization Test
**Std. Deviation: Standard Deviation
***FMD: Foot and Mouth Disease
Table 3: The comparison of antibody titers differences between three groups vaccinated with ecthyma and FMD on 60th days.
| Antibody titers detection methods* | |||||||||||
| VNT | LPB ELISA | ||||||||||
| FMD Serotypes*** | Vaccinations | Animal Number (n) |
Mean Value |
Std. Deviation** | Mean Rank | P value | Animal Number (n) |
Mean Value |
Std. Deviation | Mean Rank | p value |
|
FMD O-TUR-14 |
FMD O-TUR-14 | 6 |
51.55 |
36.09 |
10.83 |
0.89 |
6 | 79.72 | 56.57 | 12.00 | 0.173 |
| FMD O-TUR-14 + Ecthyma simultaneously | 6 |
10.08 |
6 |
10.92 | |||||||
| FMD O-TUR-14+ Ecthyma combine | 6 | 8.70 |
6 |
7.25 |
|||||||
|
FMD A-TUR-14 |
FMD A-TUR-14 | 6 |
91.22 |
64.52 |
10.25 |
0.75 |
6 |
123.78 |
82.35 |
11.25 | 0.213 |
| FMD A-TUR-14 + Ecthyma simultaneously | 6 | 10.01 |
6 |
10.77 |
|||||||
| FMD A-TUR-14+ Ecthyma combine | 6 | 9.17 | 6 |
7.58 |
|||||||
|
FMD Asia-1 TUR-14 |
FMD Asia -1 TUR-14 | 6 | 125.1 | 104.18 | 12.25 | 0.133 | 6 |
156.44 |
70.18 |
11.33 |
0.36 |
| FMD Asia -1TUR-14+ Ecthyma simultaneously | 6 | 9.92 |
6 |
10.58 |
|||||||
| FMD Asia-1 TUR-14+ Ecthyma combine | 6 | 7.33 | 6 | 8.87 | |||||||
* LPB ELISA: Liquid Phase Blocking-ELISA; VNT: Virus Neutralization Test
**Std. Deviation: Standard Deviation
***FMD: Foot and Mouth Disease
Figure 1c: Virus neutralization antibody titers against FMD (foot and mouth disease) virus type Asia 1.
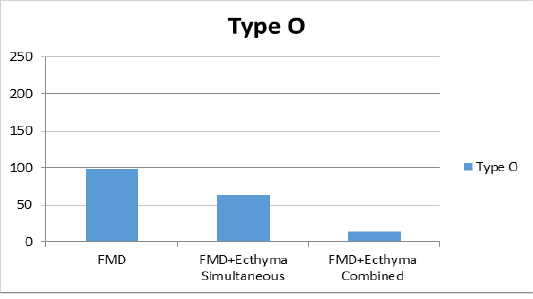
Figure 2a: Liquid phase blocking-ELISA antibody titers against FMD (foot and mouth disease) virus type O.
Figure 2b: Liquid phase Bbocking-ELISA antibody titers against FMD (foot and mouth disease) virus type A.
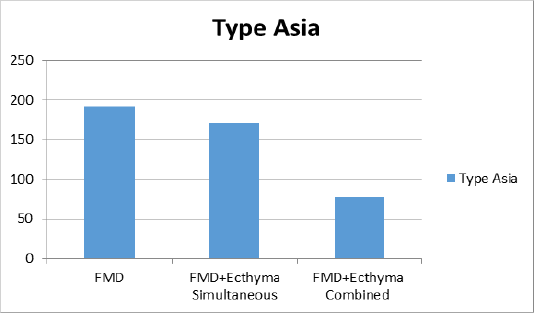
Figure 2c: Liquid phase blocking-ELISA antibody titers against FMD (foot and mouth disease) virus type Asia 1.
combined vaccine (Figure 2a). For type A, the group vaccinated with only the FMD vaccine showed a titer of 1:147 and mean titer was determined as 1:112 in group of simultaneous FMD and ecthyma vaccines, and 1:47 in vaccinated with both vaccines combined (Figure 2b). In the serotype Asia1, only the FMD vaccine group showed a titer of 1:192, while the mean titer was 1:170 in the case of simultaneous FMD and ecthyma vaccines and 1:77 in the combined vaccine (Figure 2c).
Statistical Analysis
The higher antibody levels were detected in sheep with the single FMD vaccinated group compared with the other two groups. Antibody titers in sheep with the simultaneous vaccinated group were numerically decreased as compared to the single FMD vaccinated group. Although statistically differences were not found in the antibody titers in sheep vaccinated with simultaneously or combined group as compared to the single FMD vaccinated group on day 30 as well as 60 (p >0.05), (Table 2 and Table 3).
DISCUSSION
Vaccination plays a very important role in the protection of animals against FMD. The low immunity level of available FMD vaccines and the short duration of this immunity led researchers to try new vaccines production. Although many studies have been studied on this area, unfortunately the results any of them have not been implemented. However, several studies have shown that the strength and duration of immunity can be increased by adding a number of immunomodulators without altering the vaccine formulation (Xiao et al., 2007; Çokçalışkan et al., 2016). Besides, different vaccines can be administered simultaneously at different body regions or within the same injector/formulation (combined) for cost effective, labor efficient use and better immune response. Numerous publications are available on the safe use of multiple FMD vaccines together with a number of bacterial and viral agents (De Clercq et al., 1989; Trotta et al., 2015). In most of these studies, it has been proven that the agents used together do not adversely affect the response to each other.
It has been reported that protective neutralizing antibody titers (cut-off titers) against A and O serotypes should be at least 1.20 and 1.04 in cattle vaccinated with homologous FMD vaccine containing serotypes A and O at a protective dose of 6PD50 (Çokçalışkan et al., 2016). In our study, VNT and ELISA antibody titers were found to be higher than cut-off value in all 3 groups of animals vaccinated with trivalan FMD A, O, Asia 1 serotypes alone, and vaccinated with FMD A and O serotypes used together with ecthyma vaccine and antigen. This results showed that the ecthyma inactive antigen reduces the antibody response against the FMD vaccine. Our results are concordant with another study (Kruse and Weber, 2001). That study demostrated that the presence of ORF virus impeded T-cell activation in-vitro. Moreover, ORF antigen induced apoptosis in the population of monocytes and antigen presenting cells. Similar results were obtained in another study in which vesicular stomatitis and FMD virus were simultaneously administered. The interference mechanism was reported to be responsible for the low antibody levels observed in animals vaccinated with two vaccines (Castaneda et al., 1976).
Poxviruses are known to activate a wide variety of cellular proteins to escape host immune defense, including viral interleukin 10 (IL-10), granulocyte-macrophage colony stimulation factor, and IL-2 suppressing protein. In addition, the virus also causes the secretion of IL-4, an anti-inflammatory interleukin. IL-10 is the most important anti-inflammatory cytokine and suppresses cellular responses in various ways (Friebe et al., 2004). Data obtained in this study are thought to be due to the immunomodulatory effect of ecthyma virus described above. IL-10 has also been shown to cause persistent infection by this mechanism in animals infected with FMD virus (Zhang et al., 2015). Ecthyma virus has been shown to stimulate the release of inflammation-suppressing cytokines as well as inflammatory cytokines. It has also been reported to have antiviral and anti-fibrotic effects (Paulsen et al., 2013).
Conclusion
The immunostimulant effect of ecthyma vaccine could not be determined on the antibody response against FMD in sheep with the combined or the simultaneous administration of two vaccines (inactivated ecthyma virus antigen with FMD vaccine). Although we could not find significant statistical differences between the FMD and FMD with ecthyma simultaneous or combined application, thus, the usage of these two vaccines together is not suggested at all.. Further studies should be conducted to reveal the mechanism behind this. Thus, it is contemplated that the immunogenic mechanism of the FMD vaccines can be revealed and more effective FMD vaccines can be developed.
Acknowledgement
This project was supported by the General Directorate of Agricultural Research and Policies (TAGEM) affiliated to the Ministry of Agriculture and Forestry, Turkey. TAGEM / HSGTAD /16/A07/P02/68.
Conflict of interest
The authors declare that they have no conflict of interest.
Author Contributions
VG and FS designed the experiments, ES, SU, MK and OK were prepared FMD and ecthyma vaccine mixers. VG, FS, CÇ, AA, BS carried out the all experiments. VG, BS and CÇ analyzed the data and VG wrote the paper.
REFERENCES