Journal of Animal Health and Production
Research Article
Effect of the Fowl Tick Argas persicus (Oken, 1818) (Acari: Argasidae) Infestation on the Health of Baladi Chicken in Jeddah, Saudi Arabia
Amal M. Alzahrani1*, Nada O. Edrees2
1Department of Biology, Faculty of Arts and Science in Almandaq, Al Baha University, Saudi Arabia; 2Department of Biology, Faculty of Science, University of Jeddah, Saudi Arabia.
Abstract | The fowl tick Argas persicus is an obligate ectoparasite, transmit different diseases and cause health problems such as body weight loss and toxicosis. The objective of this study was to evaluate the health status of chicken infested by A. persicus through the estimation of some blood parameters and body weight. In this study, the health of local (Baladi) chickens heavily infested twice by A. persicus (20 ticks per bird each time) was assessed. The hemogram parameters such as hematocrit (Hct), red blood cells (RBC), white blood cells (WBC), and hemoglobin (HGB) and body weight (BW) were estimated to evaluate the health damage of infested birds. During four weeks of infestation, investigations were analyzed for experimental and control group chickens. At the end of the fourth week infested groups lost 19% of their body weight, while there were 52%, 32%, 52% and 38% decrease in Hct, WBC, RBC and HGB levels respectively in chickens of infested group as compared to control group (P < 0.01). By the end of the experiment, 55% of all infested birds died. In conclusion, the A. persicus ticks caused significant weight loss and deteriorate blood picture of Baladi chickens. Hence, it is suggested that A. persicus may cause huge economic losses to poultry industry, therefore, integrated tick control program should be implemented to improve chicken productivity.
Keywords | Argas persicus, Argasidae, Fowl tick, Baladi chicken, Health, Blood variables
Received | September 20, 2019; Accepted | November 02, 2019; Published | February 19, 2020
*Correspondence | Amal M Alzahrani, Department of Biology, Faculty of Arts and Science in Almandaq, Al Baha University, Saudi Arabia; Email: amlzahrani@gmail.com
Citation | Alzahrani AM, Edrees NO (2020). Effect of the fowl tick argas persicus (oken, 1818) (acari: argasidae) infestation on the health of baladi chicken in jeddah, saudi arabia. J. Anim. Health Prod. 8(1): 13-18.
DOI | http://dx.doi.org/10.17582/journal.jahp/2020/8.1.13.18
ISSN | 2308-2801
Copyright © 2019 Alzahrani and Edrees. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Ectoparasites cause serious health damage to the host (Edworthy et al., 2019). Most external parasites such as mites and ticks are hematophagous (Boyd EM, 1951). Infested birds suffer from weight loss of about 711 g per bird and decrease egg production at the rate of about 66 eggs per bird in one year of infestation (El-Kifl et al., 1973). Among all ectoparasites, Argas persicus is one of the most important ectoparasites that cause severe blood loss and large morbidity affecting economical production of poultry (Lisbôa et al., 2009; Phulan et al., 1984). Fowl tick is known as a vector of some significant parasitic, viral and bacterial diseases giving this parasite its medical and veterinary value in tropical and subtropical regions (Hobbenaghi et al., 2016). Infested birds are irritated, have poor appetite, diarrhea and cause tick paralysis in chickens (Mcintosh and McDUFFIE, 1956; Shah, 2004).
Laboratory experiments and field observation of birds infested by ticks revealed that chronic exposure to such ectoparasites may cause a significant reduction of hematocrit level and erythrocyte sedimentation rate (Heylen and Matthysen, 2008). Moreover, chickens infested by multiple ectoparasites show major depletion of hemoglobin, red blood cells, hematocrit level in one hand, and increase of total white blood cells, eosinophil, and heterophil on the other hand (Al-Saffar and Al-Mawla, 2008). Blood samples collected from hens infested by mites show a significant reduction in packed cell volume, lower mean cell hemoglobin, and higher mean cell volume (Kilpinen et al., 2005). In addition to ectoparasites’ infestation, a complicated infection with bacteria and fungi is one more likely reason to increase the total white blood cells and atypia (Cotter, 2014).
The present study was carried out to investigate the effect of A. persicus infestation on the health of Baladi chicken, shown by hematological changes in blood components and body weight. The study hypothesizes that A. persicus infestation may lead to severe health problems. Heavy infestation causes anemia and death as a result of hematocrit (Hct), red blood cells (RBC), hemoglobin (HGB) reduction in blood as well as a decrease of body weight (BW). However, the increase of white blood cells (WBC) or leucocyte concentration may indicate a pathogenic state of health.
MATERIALS AND METHODS
Chicken Housing and Feeding
The study was approved by the Al Baha University, Saudi Arabia and all study protocols were in line with international ethical guidelines of animals. Twenty-five hens of Baladi (local) Chicken at around 14 weeks of age were purchased from a local bird market located in the southern area of Jeddah, Saudi Arabia. The parasite-free pullets were physically screened for fowl ticks and other ectoparasites. They were randomly distributed into 4 infested groups (A, B, C, D), and one control group (CON) with 5 birds in each group. All A. persicus infested groups were isolated in one concrete room-sized 4 m × 5 m, while the control group was placed in a separate room away from the infested groups.
Four isolated chicken pens measuring 120 cm × 120 cm × 100 cm were placed in the room of infested groups. Pens were made of wood and transparent thick plastic walls for better viewing and observation during the day and night. All building materials of the pens were fixed with nails and sealed securely with silicon glue. Each pen was supported with ceiling ventilation to control air and temperature inside the pens (Figure 1). There were also hiding places inside each pen providing the right shelter for ticks to hide after receiving a blood meal from the host during the night. Each hiding place was made of a wooden box sized 50 cm × 20 cm × 10 cm and opened from the base. Moreover, tick traps were made of cardboard size 10 cm x 7 cm × 4 mm and fixed inside the hiding places (Figure 2). The traps inside the hiding places help to evaluate the intensity of infection and to estimate the tick population every week.

Figure 1: Special pens made for the experiment
Rooms were supported with air conditioning and fans to maintain the temperature at 30 ± 5 ºC and relative humidity (RH) at 35 ± 5% (Hafez et al., 1972; Hassanali et al., 1989; González-Acuña et al., 2010). The light was provided by two LED 40W light bulbs and kept on for about 14 hours a day in each room. The hens were fed (Fakieh Feed by Fakieh Group, Jeddah, Saudi Arabia) commercial poultry feed, purchased from a local market consisting of 58% maize, 35% soybean, 1.5% limestone, 1.5% calcium and phosphorus, 1% vitamin, 1% mineral salts, and 2% vegetable oils. Water was provided directly from the tap.

Figure 2: Ticks inside tick traps
Infestation Technique
The ticks of A. persicus used in this study were collected from several local poultry farms in Jeddah Governorate, Saudi Arabia. They were identified using tick identification key (Walker et al., 2003). These ticks were found in the cracks, crevices, below the feed trough, on birds and in the chicken house. They were also trapped using cardboard tick traps (Figure 2). Ticks were saved in isolated rearing tubes (tubes with a small hole in every stopper) that will be a house to store live ticks. Holes must be covered with small pieces of white clothes from inside the tube and should be secured with any fitting rubber (Figure 3). Plug holes allow necessary ventilation and the fabric for preventing larvae from escaping outside the bottle while letting the air inside the bottles.

Figure 3: Rearing tubes
Two groups of A. persicus were introduced twice to the infested groups. Each group of ticks consisted of approximately 400 adults, nymphs and larvae of A. persicus. The first infestation by ticks took place on the first day of the experiment. The parasites were distributed almost equally between the infested groups (A, B, C, D). Around one hundred ticks were placed inside each isolated pen. Ticks were had given enough time to hide during the day before birds were placed inside the pens to spend the whole night there. Every morning, hens were allowed to get out of pens and stay inside the room to eat and drink. After one week of infestation, it was noticed that the number of ticks inside tick traps and hiding places was decreased. However, it was decided to introduce the second group of ticks on the seventh day. Similarly, the second group of ticks was divided equally between the four pens, one hundred ticks for each pen.
At the end of every week, pens were opened and cleaned. Ticks were found hiding in the traps and clustered in the roof and corners of the hiding places (the wooden boxes) inside the pens (Figure 4). All different life stages of A. persicus were collected and then grouped to estimate the population of ticks on days 7, 14, 21, 28.
Collection and Analysis of Blood Samples
During four weeks of the experiment, measurements of changes with bird specimens were taken in five variables reflecting health condition of each experimental group: Hematocrit (Hct), White blood cell (WBC), Red blood cell (RBC), Hemoglobin (HGB), and body weight (BW). The body weight of every hen was measured once a week. Blood samples of all birds were collected from wing veins on days 7, 14, 21 and 28 of the experiment. Two ml (±0.5 ml) of blood were saved immediately to 3 ml EDTA tube lavender-top. Samples were labeled and then refrigerated at 8 to 12° C. They were sent to the laboratory for hematological analysis within 24 to 48 hours. Cell-DYN 3500 Hematology Analyzer (Abbott Diagnostics Division, Mountain View, CA) was used to perform CBC (Complete Blood Count) of 100 blood samples from 25 hens in 4 weeks. This simple type of automated hematological analysis has been proved to be a sensitive and reliable tool to measure blood cell parameters specifically for broiler chicken (Post et al., 2003).
Statistics
Data were analyzed using the IBM SPSS Statistics v.23 software (2015) by T-test. Significance was set at P < 0.01.
RESULTS
Blood Variables and Body Weight
The summary of weekly blood investigation and body weight of four infested groups and one control group are shown in Table-1. Significant reductions (P < 0.01) of all hematological values (Hct, RBC, WBC, HGB) and body weight (BW) of all infested groups were recorded during four successive weeks. The blood values and BW decreased gradually from week 1 to week 4 (see Figure 5 a-e). According to the table 1, the infested groups lost about 19% of their body weight in four weeks of A. persicus infestation. The Hct level dropped 52% by the end of week 4. The percentage of RBC reduction was 52%. The HGB level decreased by 38% compared to the control group. The WBC level was 32% less than the control group. However, 15 clotted blood samples were rejected in weeks 2, 3 and 4. Eleven blood samples were missing due to host mortality in weeks 3 and 4. It was very hard to collect blood samples from infested birds in weeks 3 and 4. The wing vein didn’t show clearly to draw blood samples from almost all infested birds. Most blood samples that have been taken in week 4 were clotted immediately after collection from infested groups.
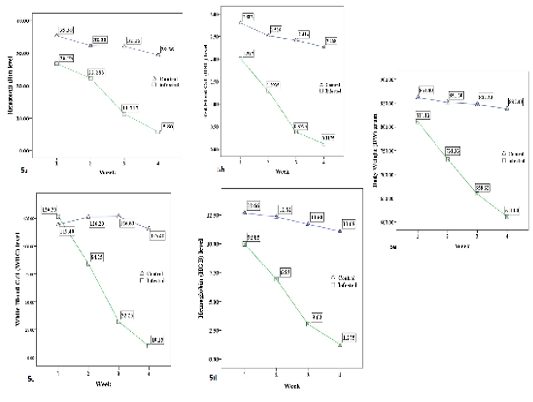
Figure 5: The average (a) Hematocrit %, (b) Red blood cells × 1012/L, (c) White blood cell × 109/L, (d) Hemoglobin g/dl, and (e) Body weight /g level of the control group and infested groups during 4 weeks trial period.
Table 1: Mean (± SE) hematological variables and body weight of control and A. persicus infested Baladi chickens.#
|
Health parameters& |
Infested group (Percent difference as compared to Control group) | Control group |
Normal range$ |
| Hematocrit % |
15.4 ± 2.1 (-52%)* |
32.0 ± 1.2 | 30-49 |
|
RBC (× 1012/L) |
1.18 ± 0.15 (-52%)* | 2.47 ± 0.12 | 2.5-3.9 |
|
WBC (× 109/L) |
82.5 ± 9.5 (-32%)* |
121.6 ± 2.8 | 1.9-9.5 |
| HGB (g/dl) |
7.3 ± 0.63 (-38%)* |
11.8 ± 0.34 | 10.2-15.1 |
| BW (g) | 686.9 ± 18 (-19%)* | 849.0 ± 5.0 | 800-900 |
# All values were presented as means of four weeks.
& RBC: Red blood cells; WBC: White blood cells; HGB: Hemoglobin; BW: Body weight.
$ The Normal range was adapted from (Samour, 2006).
* Showing significant difference as compared to control group at P < 0.01.
Tick Population
To estimate the tick population, pens were opened and ticks were collected from hiding places and tick traps at the end of every week of the experiment. The infestation was started by 20 ticks per bird (total of 400 ticks) in week 1. At the end of the first week, it was noticed that the number of ticks decreased about 35% (260 ticks). Some ticks were found dead inside the hiding places and others might be eaten by the birds. Some missing parasites might be still attached to the host specially unfed larvae. For these reasons, it was decided to introduce another wave of A. persicus (400 adults, nymphs and larvae) at the beginning of week 2 to the infested groups. The infection rate in this case should be almost double (40 ticks per bird) if we ignore the missing parasites. By the end of the second week, the total number of ticks was 620 ticks decreasing about 22.5%. At the end of week 3, hundreds of new hatched (unfed) larvae were found in hiding places and tick traps. At the end of week 4, the estimated number of population was 379 adult, 82 nymph, and 600 engorged larvae. The total number of ticks increased almost 71% compared to the total number of ticks in week 2.
Mortality
By the end of the experiment, eleven (55%) out of 20 infested birds were dead. No mortality was recorded in weeks 1 and 2, and no mortality was recorded within the control group. However, during the second week of the experiment, 2 birds belong to the group samples (C & D) of the infested groups were suffering from severe anemia. The level of Hct and RBC for one sample were found very low (eg, Hct = 10.6%). The hosts’ mortality began at week three. The two previous sick birds in week 2 were found dead in week 3 (day 16 and 20). By the end of week four, 9 additional infested birds were found dead. They were already suffering from anemia, low body weight, losing appetite and general weakness. It was decided to terminate the experiment by the end of week 4 for several reasons. The increase in mortality specifically in week 4 made it difficult to continue with less than 50% of infested groups against the increasing population of A. persicus. For example, groups B and C were left with only two samples in each group at the end of week 4. The hens survived in the infested groups were weak, tired, inactive, and anemic (Figure 6). It was very difficult to collect sufficient volume of blood samples especially in week 4. Most blood samples collected in week 3 and 4 were clotted and didn’t show the accurate hematological results.

Figure 6: Infested birds are weak and inactive
DISCUSSION
Although many argasid ticks tend to have fewer blood meals than ixodid ticks, the larvae of A. persicus can increase their body weight up to 20 times in a fairly longer feeding time before molting to a nymphal stage (Lindquist and Wu, 2016). When A. persicus infestation intensity was 3.25 per bird, one tick could suck an amount of 18.57 mg of blood in one day, and a bird could lose about 0.06 g of blood every day (Khan et al., 2001). This very high consumption of blood from the host leads to a sudden reduction in blood level and blood components (Anemia).
Packed Cell Volume (Hematocrit) is an essential hematologic test. It is a basic and reliable method to estimate the level of RBC in a blood sample (Samour, 2006). Several factors are known to cause Hct reduction in blood: Low ambient temperatures, hematophagous ectoparasites, infection with haematozoa, and nutritional and environmental stress (Shlosberg et al., 1992; Potti et al., 1999; Dusek et al., 2004; Piersma et al., 2000). In the current study, the Hct level of all infested groups was always lower than the level of the control group. Similar results have been found in the effect of tick parasitism on the health status of a passerine bird (Heylen and Matthysen, 2008). However, a huge reduction in the Hct level of all infested groups was recorded in week 3 (50% lower than week 2). This prominent reduction of the Hct level was accompanied by host mortality in weeks 3 and 4. Unlike, Heylen and Matthysen (2008) found no correlation between the number of ticks and the degree of Hct reduction when they investigated the health status of a passerine bird infested by the sheep tick (Ixodes ricinus). The current study found that the presence of new tick larvae in week 3 and the introduction of new ticks in week 2 (high intensity) could be the reason behind the great reduction of Hct. Clinically, we reject any effect due to blood withdrawn for the hematological test, and it is safe to take up to 10% (1.5-2.7 ml) from each bird for lab purposes (Samour, 2006). Furthermore, the same thing can be said to explain the reduction of RBC and HGB since Hct is needed to measure other blood constituents such as hemoglobin concentration and the Red Cell Absolute Values (Samour, 2006).
Heterophils are essential in the avian immune system. They can identify and terminate microbial threats in the blood (Genovese et al., 2013). The total number of WBC may vary among avian species (Samour, 2006). The WBC of both infested groups and the control group were above the normal level (leukocytosis). The increasing number of heterophils in the blood may cause extreme leukocytosis (Jakubas et al., 2013). Many reasons can cause avian leukocytosis: stressful environment, animal transport, microbial infection, housing system and temperature stress (Cotter, 2014; Scope et al., 2002; Harmon, 1998; Campo et al., 2008). Both infested groups and the control group were suffering from extreme leukocytosis. Similar results have been reported by Cotter (2014) when assessed the stress of caged hens. However, our results here are different from Heylen and Matthysen (2008) as they found no effect on leucocyte concentration. This could be a result of different intensity and duration of infestation, avian species, and tick species.
The physical condition of the infested bird was constantly exposed to intensive pressure by the ticks. Such a stressful condition caused by heavy tick infestation can affect the welfare of the birds (Wikelski and Cooke, 2006). The body of the host had to produce new blood and restore skin lesions caused by larvae infestation. The infested birds had to spend much time removing parasites from the skin, grooming, head-scratching, head shaking and end up with slow movement and depression (Kilpinen et al., 2005). The hens in both the control group and infested groups, lost body weight after they were housed in the new environment (Emery et al., 1984). However, the reduction of BW in infested groups was significantly greater compared to the control group. Similar results have been found in previous studies (Kilpinen et al., 2005; Dube et al., 2010). Unlike Heylen and Matthysen (2008), the body weight of all infested groups decreased even with a medium-low tick infestation in week 1.
Tick-borne infectious diseases transmitted by A. persicus such as fowl spirocheatosis caused by Borrelia anserina could be another factor for host mortality (Aslam et al., 2015). Although anemia and fowl spirocheatosis share some common symptoms (e.g. weakness and loss of appetite), no diarrhea have been recorded with birds in the current study (Ataliba et al., 2007). However, infested groups might be suffering from both fowl spirocheatosis and anemia at the same time, and this could be the reason behind high mortality in this study. Further investigations of fowl spirocheatosis in Baladi chicken should be conducted.
Conclusion
In summary, A. persicus infestation causes serious health problems in chickens. In addition to viral and bacterial diseases caused by fowl ticks, anemia could be the most direct health risk associated with tick infestation. Severe reduction of some important blood elements (Hct, RBC, WBC, HGB), as well as body weight loss, is the absolute result of such infestation.
ACKNOWLEDGEMENTS
Authors thank Albaha University for its unlimited support and help to fund this study.
CONFLICT OF INTEREST
There is no conflict of interest.
AUTHORS CONTRIBUTION
All authors contributed equally.
REFERENCES






