Journal of Animal Health and Production
Research Article
Using of Rapid ELISA as an Alternative Method for Evaluation of Canine Parvo Vaccines
Mohamed Samy Abousenna*, Amal AM, Heba MG Abdel Aziz, Waleed Barghooth, Nermeen G Shafik
Central Laboratory for Evaluation of Veterinary Biologics (CLEVB), Egypt.
Abstract | Canine parvovirus (CPV) vaccine is recommended to be used for dogs especially in young age. Vaccination plays an important role in reducing death rates, preventing clinical cases and controlling the spread of virus. Evaluation of live attenuated CPV vaccine requires in vivo and in vitro techniques. In the present work, we investigated the sensitivity of the SNAP Parvo test (rapid ELISA) in comparison with immunofluorescence technique (IFT) using reference canine parvovirus to determine the CPV content in live attenuated CPV vaccine. Ten batches of live attenuated CPV vaccine were tested by SNAP parvo test and IFT, on the other hand the vaccine batches were inoculated in ten dog groups and samples of their sera were collected after 14 days from booster dose to evaluate the immune response using serum neutralization test (SNT). It was found that the sensitivity of SNAP Parvo test at virus dilution of 4 and 5 was 90%. The SNT showed antibody titers for the sera of vaccinated puppies not less than 32 (1.5 log10 TCID50) for eight batches which were correlated with SNAP Parvo test and IFT. These findings suggest the possibility of using SNAP Parvo test for detection of virus titer in live attenuated CPV vaccine. It could be used as a rapid primary test for evaluation of unsatisfactory vaccines, in order not to continue the following evaluating procedures, subsequently minimizing the experimental animal use, effort and consumed time and cost.
Keywords | Canine Parvovirus, Rapid ELISA test, Live attenuated Canine Parvovirus vaccine, Vaccine evaluation.
Received | September 20, 2019; Accepted | November 02, 2019; Published | February 01, 2020
*Correspondence | Mohamed Samy Abousenna, Central Laboratory for Evaluation of Veterinary Biologics (CLEVB), Egypt; Email: mohamedsamy2020@hotmail.com
Citation | Abousenna MS, Amal AM, Aziz HMGA, Barghooth WM, Shafik NG (2020). Using of rapid elisa as an alternative method for evaluation of canine parvo vaccines. J. Anim. Health Prod. 8(1): 8-12.
DOI | http://dx.doi.org/10.17582/journal.jahp/2020/8.1.8.12
ISSN | 2308-2801
Copyright © 2020 Abousenna et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Canine parvovirus (CPV) is responsible for acute and sometimes fatal enteritis in dogs. The virus, which first appeared in late 1978 probably arose from a very closely related virus in cats, feline panleukopenia virus through a small number of mutations in the single capsid protein (Tattersall et al., 2005). CPV is a non-enveloped virus of the autonomously replicate Parvoviridae family. This small icosahedral virus has a single-stranded DNA genome (5.2 kb) with two major open reading frames, one encoding the nonstructural proteins NS1 and NS2, and the other encoding the capsid proteins VP1 and VP2 (Nakamura et al., 2004).
Modified live virus (MLV) vaccines are widely used. These vaccines are highly effective and prepared by using either the original type CPV-2 or its variant CPV-2b (Decaro et al., 2014). These may protect dogs against parvovirus infection, and almost completely safe, as post vaccinal reactions are very rarely observed. There is study showed that most dogs developing parvovirus-like diarrhea after vaccination were infected by the field virus alone, thus ruling out any reversion to virulence of the vaccine viruses (Greenwood et al., 1995).
The primary causes of failure of CPV vaccination are interfering levels of maternally derived antibodies (MDA) that are transmitted by bitches to their offspring through colostrum and, at a lesser extent, milk. Thus, in order to avoid the interference with MDA active immunization, vaccines should be administered to pups only after weaning (Lida et al., 1990).
Vaccination stimulates both humoral response via antibody production and cellular responses via B and T lymphocytes (Day et al., 2012). Therefore, measurement of serum antibody titers can be a helpful tool for determining the efficacy or need for vaccination (Tizard and Ni, 1998) and for making population management decisions in farm animal (Newbury et al., 2009; Lecher et al., 2010). Serum CPV antibody titers can be measured by ELISA, indirect fluorescent antibody assays (IFA) or by hemagglutination inhibition (HI) or serum neutralization test (SNT) (Twark and Dodds, 2000). In Central Laboratory for Evaluation of Veterinary Biologics (CLEVB) tissue culture titration for live attenuated canine Parvovirus vaccine using Immunofluorescent antibody technique is conducted, and the sera of vaccinated puppies were tested using SNT (unpublished data). In the current study an alternative evaluation for virus titer of live attenuated canine parvovirus vaccine was applied using rapid SNAP Parvo test in comparison with tissue culture titration using immunofluorescence technique (IFT). Regarding the SNT results, using of SNAP Parvo test will be useful to minimize the time of evaluation and experimental animals, and also cut the need of professional staff and laboratory facility.
MATERIAL AND METHODS
Experimental Material
Ten batches of live attenuated canine Parvovirus vaccines were delivered to Central Laboratory for Evaluation of Veterinary Biologics (CLEVB), Abbassia-Cairo. These batches had been evaluated in the last year for sterility, safety and potency. Madin-Darby Canine Kidney (MDCK) cells were supplied by Strain Bank at CLEVB. The cells were used for IFT, SNT, vaccine titration and identification. Canine parvovirus tissue culture was adapted on MDCK with titer of 6 log10TCID50 /ml. This virus (positive control) was used in IF technique, sensitivity test for SNAP Parvo test and SNT for serum samples of vaccinated pups (Ingy, 2018). Viruses of rabies (Edries, 1994), canine distemper (Guirguis, 1991), and canine adeno (Khodeir et al, 2003) were used (negative control) in specificity test for SNAP Parvo test. All these viruses were supplied by the Department of Pet Animal Vaccine Research (DPAVR), Veterinary Serum and Vaccine Research Institute (VSVRI), Abbasia, Cairo.
Immunofluorescence Technique (IFT)
Titration and identification of the tested viral vaccines and positive control parvovirus were performed by IF technique, using MDCK cells tissue culture coated microtiter plate as described previously (USDA-APHIS-CVB 2014). The 50% fluorescent antibody infective dose (FAID50) of the test vaccines and positive control were calculated according to Sperman method (Spearman-Kärber, 1908). This test was used for evaluation of vaccine batches and parvovirus in sensitivity test.
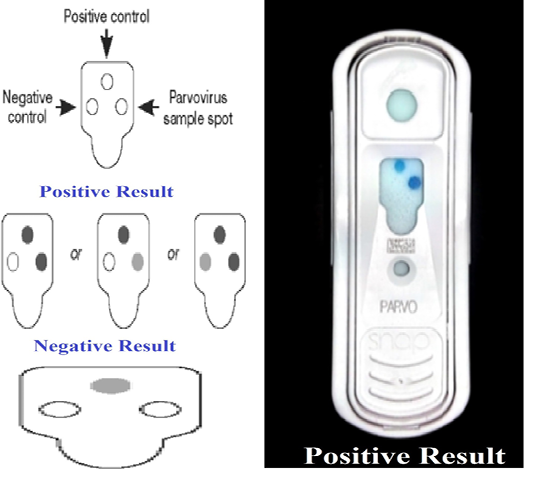
SNAP Parvo Test (Rapid ELISA)
SNAP parvo test (IDEXX Veterinary Diagnostics-IDEXX, USA) kit was used to detect canine parvovirus in vaccines and to determine its sensitivity and specificity. The test was carried out according to Thrusfield, (2007), on canine parvovirus obtained from the Department of Pet Animal Vaccine Research (DPAVR), Veterinary Serum and Vaccine Research Institute (VSVRI), Abbasia, Cairo. The CPV virus was diluted serially tenfold (10-1 up to 10-8), the minimal concentration of virus showing positive results with SNAP parvo test was determined and compared the results with that detected by virus titration on tissue culture using IFT. The cut off value was calculated by ROC curve using SPSS IBM version 21. Each virus dilution was tested by 10 strips of SNAP parvo test for detection of the sensitivity percentage.
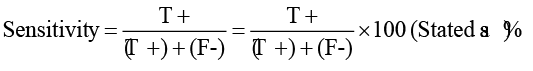
T+: True Positive, T-: True Negative, F+: False Positive, F-: False Negative
In order to verify the specificity of SNAP parvo test against CPV only, some other viruses like, rabies, canine distemper, and adeno virus were also tested by SNAP parvo test.
Animals
Thirty puppies of native breed (age: 50-60 days, three weeks post weaned) free from antibodies against canine parvovirus, were made available by CLEVB. They were used for evaluation of the ten batches of live attenuated parvovirus vaccine (three puppies/each batch) by inoculation each dog with one dose then booster dose after 21 days, Blood samples were collected from vaccinated dogs after 14 days from booster dose, then tested by SNT.
Serum Neutralization Test (SNT)
It measures the humoral immune response against CPV in the sera of vaccinated puppies by live attenuated canine parvovirus vaccine batches. SNT was carried out using the microtiter technique according to Bonnie et al. (1983). The antibody titer was calculated as log 10 TCID50 according to Reed and Muench, (1938).
RESULTS
Detection of the sensitivity of the SNAP parvo test using standard positive CPV (positive control) in comparison with virus titration on tissue culture using IFT showed that the minimal concentration of virus that showed positive result with SNAP parvo test with cutoff value 4.5 and sensitivity percentage 90 as shown in Table 1. The specificity showed 100% result when the SNAP parvo test was used
Table 1: Sensitivity of SNAP-Parvo test using standard positive canine parvovirus in comparison with immunofluorescence technique (IFT)
| Item | Tested dilutions of standard positive canine parvovirus | ||||||||
| -1 | -2 | -3 | -4 | -5 | -6 | -7 | -8 | ||
| *IFT | +ve | +ve | +ve | +ve | +ve | -ve | -ve | -ve | |
|
**SNAP test (10 strips)
|
T+ | 10 | 10 | 10 | 9 | 9 | 0 | 0 | 0 |
| T- | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | |
| F- | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | |
| F+ | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | |
| Sensitivity % of SNAP | 100 | 100 | 100 | 90 | 90 | 0 | 0 |
0 |
|
*-ve result of IFT refer to normal cells with no detectable virus while +ve refer CPE in tissue culture with detectable virus green spots.
**T+: True Positive, T-: True Negative, F+: False Positive, F-: False Negative
Table 2: Evaluation of the canine parvovirus titer in live attenuated canine parvovirus vaccine batches using immunofluorescence technique (IFT) and SNAP test (rapid ELISA).
|
Vaccine batches No. |
IFT |
SNAP test |
| +ve titer | ||
| 1 | 4 | 4 |
| 2 | 3 | 3 |
| 3 | 4.5 | 5 |
| 4 | 3.5 | 4 |
| 5 | 3 | 3 |
| 6 | 2 | 2 |
| 7 | 3 | 3 |
| 8 | 1 | 1 |
| 9 | 3 | 4 |
| 10 | 4 |
4 |
against canine distemper virus, canine rabies virus and canine adeno virus (negative control samples).
Evaluation of the CPV titer in live attenuated CPV vaccine batches using IFT and SNAP, showed that virus titer was not less than 3 log10 in eight vaccine batches as shown in Table-2 and Figure-1.
Evaluation of humoral immune response in the sera of vaccinated puppies was detected by using SNT and the results of the all ten vaccine batches were presented in Table 3. It was showed that, eight batches (1, 2, 3, 4, 5, 7, 9 and 10) reached permissible (protective) limit (1.5 log10) while two batches (6 and 8) did not show protective titer.
DISCUSSION
An effective way to prevent the infection in domestic dogs against CPV infections is vaccination (Greene and Schultz, 2006). Studies on live attenuated CPV vaccines, using limited number of animals, reported increasing antibody titers (Spencer and Burroughs, 1992; van Heerden et al., 2002), which correlates well with protection against CPV disease in domestic dogs (Schultz, 2006).
The present study was aimed to evaluate the existing imported commercial live attenuated CPV vaccine batches in domestic puppies for its efficacy using SNT and tissue culture virus titration using IFA, to develope an alternative indirect potency test instead of IFA tissue culture virus titration with consideration and correlation to SNT. Sensitivity of the rapid SNAP parvo test was evaluated by comparison with the traditional used technique in this study. The rapid SNAP parvo test showed a diagnostic sensitivity of 90% at Cutoff value of 4.5 for detection of CPV compared with IFA tissue culture virus titration. While the specificity showed result 100% when the SNAP parvo test used against canine distemper virus, canine rabies virus and canine adeno virus. These results are in agreement with other workers (Schultz et al., 2008) who conducted sensitivity and specificity of the SNAP® parvo test and reported 100% results for each.
We also tested the imported commercial live attenuated CPV vaccine batches using virus titration on MDCK tissue culture using IFA and SNAP parvo test. The results of virus titration complied with SNAP parvo test indicated the possibility to use SNAP parvo test as alternative
Table 3: Evaluation of the humoral immune response of vaccinated puppies with live attenuated canine parvovirus vaccine batches using serum neutralization test (SNT).
| Vaccine Batches No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|
SNT Antibody titer (Log10 TCID50) |
2.1 | 1.8 | 2.4 | 1.95 | 1.5 | 1.05 | 1.65 | 0.6 | 1.8 | 2.25 |
method. The satisfactory vaccine is tested by SNAP parvo test would be having virus titer 100.5 greater than the titer calculated by virus titration on tissue culture using IFA with consideration to the permissible (protective) limit of the virus titer which should not less than 103 TCID50/ml (CFR, 2012).
Evaluation of humoral immune response for the sera of vaccinated puppies was also carried out by SNT to detect neutralizing antibody titer. The results for the ten batches showed that eight batches reached permissible limit of CPV antibodies titer (1.5 log10) as suggested by previous workers (Bass et al., 1982; Bonnie et al., 1983), however, two batches (6 and 8) did not show protective titer (Table-3). The SNT was carried out in another study to evaluate the CPV vaccine or CPV hyper immune serum and authors detected neutralizing antibody titer of dogs inoculated by anti-canine parvovirus hyper immune serum as 1024/ml Attyat and Wafaa, (2015). Also Jayalakshmi Vasu et al. (2019) used SNT to compare immune response of two groups of vaccinated puppies, one group given single.
gle booster dose while the other given double booster dose of modified live canine parvovirus vaccine. Similarly, Sherry Glover et al. (2012) applied SNT to detect the humoral immune response of puppies vaccinated with CPV (type 2b) vaccine in comparison with the results obtained from HI technique for the same serum samples. In the current work the results of SNT for vaccinated puppies confirmed and correlated with the results obtained from IF technique and SNAP parvo test which were used in evaluation of the tested vaccine batches.
Conclusion
These findings suggest the possibility of using SNAP parvo test for detection of CPV titer in live attenuated CPV vaccine. This rapid ELISA (SNAP test) could be used as a rapid primary test for detection of unsatisfactory vaccines thus may minimize the use of experimental animals, effort and time consumed.
acknowledgements
The authors would like to thank the staff from Central Laboratory foe Evaluation of Veterinary Biologics (CLEVB), Cairo, Egypt for their technical assistance and cooperation.
conflict of interest
The authors declare that they have no conflict of interest.
authors contribution
All authors contributed according to their tasks and approved the final manuscript.
References