Journal of Animal Health and Production
A Study on Toxic Effects of Silver Nanoparticles on Reproductive Tract of Male Mice
Haider Ayad Kathem*, Mohammed Jaweed Alwan
Department of pathology and poultry disease, College of Veterinary Medicine, University of Baghdad, Iraq.
Abstract | Toxic effect of silver nanoparticles (50 nm) on the male reproductive tract of mice was tried to find out in this study. Two groups with 10 male white mice (8-10 weeks old) each were investigated. For 35 days, one group was administered orally with 0.4 mL suspension of silver nanoparticles @ 10mg/Kg of body weight while the other one was administered with the same quantity of normal saline as control. Weight of mice, its testis and spleen was measured whereas histopathology was performed with tissues from epididymis. Compared to control, a decrease in the mean body weight and weight of testis was noted while it was vice versa for spleen. A high percentage of dead and abnormal sperm along with decrease in serum testosterone concentration was observed in treated mice. Histopathology revealed abnormal arrangement, deformity, atrophy, degeneration, and necrosis of epithelial cells of somniferous tubules. The study concludes potential of silver nanoparticles to penetrate the blood-testcal barrier and induce pathological changes in the testis and deformity of the sperms with reduction in their number.
Keywords | Silver nanoparticles, Reproductive tract, Mice, Toxic effect
Editor | Asghar Ali Kamboh, Sindh Agriculture University, Tandojam, Pakistan.
Received | August 13, 2015; Revised | October 05, 2015; Accepted | October 10, 2015; Published | August 01, 2019
*Correspondence | Haider Ayad Kathem, University of Baghdad, Iraq; Email: haiderayad88@gmail.com
Citation | Kathem HA, Alwan MJ (2019). A study on toxic effects of silver nanoparticles on reproductive tract of male mice. J. Anim. Health Prod. 7(3): 85-91.
DOI | http://dx.doi.org/10.17582/journal.jahp/2019/7.3.85.91
ISSN | 2308–2801
Copyright © 2019 Kathem and Alwan. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Recent epidemiological studies have reported significant increase in the prevalence of chronic disease such as diabetes, cancer, impairment of immune system and infertility of male men largely due to decrease in quality and quantity of sperms and disorder in function of the ovary worldwide (WHO, 2012). Though etiology of these diseases are unknown, however may be related to hormonal and enzymatic disturbance that result from environmental pollution (Roger et al., 2013) contaminated with different chemical material particularly industrial products such as solvent, dioxins, pesticides and nanotechnology products (De coster et al., 2012).
Nanotechnology is widely applied in different fields including manufacturing and medicine (Kalishwarala et al., 2010). The most important of nanomaterial products are the silver nanoparticles (AgNPs) as they are highly active against bacteria, viruses, fungi and particularly drug resistant microorganisms (Sondi and Salopek-Sondi B, 2004).
AgNPS are being used in routine house-hold equipment and utilities (Maynard, 2006; Hui et al., 2009) allowing the release and availability of these particles to environment. Nevertheless, a few researches have been conducted on the toxic effects of nanoparticles on human and animal health and the environment (Johnston et al., 2010).
Research reports have revealed reproductive toxicity of nanomaterial (Yang et al., 2012) observed testicular damage in mice upon repeated exposure of carbon nanoparticles without affecting their fertility. Ema et al. (2010) demonstrated potential of nanoparticles to cross biological barrier of reproductive tract and subsequent tissue damage, reduction in number and viability of the sperms and impairment of embryo development. Kim et al. (2010) reported enlargement of testis and hepatototxicity in male rats when given 55nm nanoparticles for 13 weeks daily. For post-intravenous administration of nanoparticles, Gromadzka et al. (2012) recorded toxic effects on spermatogenesis of male rates characterized by low count of epididymal sperms, destruction of DNA of germ cells and morphological changes in the somniferous tubules.
Despite a few research reported on effect of nanoparticle on reproductive tract, there lacks data revealing the influence of silver nanoparticles on male reproductive system. Therefore, present study has been conducted determine the toxic effects of AgNPs on male reproductive organs.
Materials and methods
Silver nanoparticles: CAS number (7440-22-4); MW=107.86; Assay (99.0%); Color: grey; Radius: 50 nanometer; Shape: spherical; Expire date: November 2018; Made in china.
Method
Twenty albino mice (9 to 10 weeks old) were randomly divided into two groups of ten each. One group was administered orally with 0.4mL suspension (50nm) of AgNPs for 35 days daily. The lethal dose (LD50) used in this experiment was determined as per method described previously (Pattwat et al., 2011). Likewise same quantity of normal saline was used in control group mice.
After 35 days, all were sacrificed with the collection of blood directly from heart. Tissues specimens from testis and epididymis were fixed in 10% formalin and slides were prepared.
Measurement the Body Weight of Animals and the Weight of Internal Organs (Testis, spleen)
The body weight of each mouse was recorded at the end of experiment (35days) from ademistration with 10Mg\Kg.BW AgNPs, all experimental mice were sacrificed; then the internal organs that includes (testes and spleen) were removed, stripped from fatty tissues, and examined macroscopically and weighed under the sensitive balance, and recorded the significant difference in the weights of treated and control group.
Blood Collection for Hormonal Assay
Blood was collected immediately after anesthesia, directly from heart mice by using insulin syringe 1 ml after treated with 10Mg\Kg.BW\ AgNPs, blood samples directly transferred into a Eppendorf Tube, after that kept in refrigerator for period of time in stand position then centrifuged at 1500 rpm for 3 minutes, the serum were stored in the frozen at -20°C until hormonal analysis. Hormonal analysis for Testosterone kit, was measured by using (VIDAS) technique.
Epididymal Sperm Characteristics
A. Preparation of Eosin–Nigrosin stain solution:
B. Semen collection:
The testes were removed along with the epididymis. The caudal epididymis were separated from the testes, blotted with filter papers and lacerated to collect the semen.
Sperm Morphology
A part of sperm suspension was used for preparing smears to evaluate the sperm shape abnormalities (Wyrobek et al.,1974; Wyrobek et al., 1983).
The sperm morphology was also determined using Eosin/Nigrosin stain. To test, one drop of eosin and nigrosin was added to the suspension and were mixed by gentle agitation. One to two drops of the stained sperm suspension are placed approximately 1cm from the frosted end of a pre-cleaned microscope slide lying on a flat surface. A second slide is held in the right hand with the slides’ long edge gently touching across the width of the sperm slide and pulled across to produce a sperm smear. Two to four slides are prepared from each sperm suspension. These additional slides may be used in confirming any preparation artefacts or to have additional sperm to evaluate if sperm numbers are low. Preferably, 400 sperms were examined per animal morphologically at 400 magnification.
Morphological abnormalities were classified as amorphous head, inverted with amorphous head, hook-less, banana, and double headed, coiled with microcephaly, bent at cephalocaudal junction, bent with projecting filaments, microcephaly with tail defect and defective head with duplication of tail, and curved tail. (Narayana et al., 2002).
Sperm Viability
Sperm viability was evaluated as follows. A 20 μL of eosin and nigrosin were added into an equal volume of the sperm suspension. After 2 min of incubation at room temperature, slides were viewed by light microscope with magnification of 400. Dead sperms appeared to be pink and live sperms were not stained. In each sample, 400 sperms were counted and viability percentages were calculated (Kodama et al., 1997).
Statistical Analysis
Statistical analysis was done by using ANOVA test through SPSS program.
Results
Body Weight and Weight of Testis and Spleen
Mean of the body weight of treated mice (0.57 ± 29.80) was found to be lower than control (1.44± 35.44). Weight of right (0.004 ± 0.058) and left testis (B 0.005 ± 0.060) of treated mice revealed low weight, as compared to controlled ones (0.002 ± 0.086. Interestingly, we found
Table 1: Mean body weights, weights of the testis and spleen of control and treatment animals with AgNPs
| Groups | Body weight (gm) | Weight of right testis (gm) | Weight of left testis (gm) | Weight of spleen (gm) |
| Control males |
35.44 ± 1.44 A |
0.086 ± 0.002A |
0.086 ± 0.002A |
0.132 ± 0.005 B |
| Males treated for 35 days |
29.80 ±0.57 B |
0.058 ±0.004B |
0.060 ±0.005 B |
0.154 ± 0.007AB |
Note: All results were presented as mean ± standard error. Means showing different letters vertically indicates statistical differences (p< 0.05).
Table 2: Mean and stander error for the (serum levels of testosterone in control and treatment animals with AgNPs
| Groups | Testosterone hormone |
| Control males |
9.485 ± 1.93 A |
| Males treated for 35 Days |
1.358 ± 0.53 B |
Note: Means showing different letters vertically indicates statistical differences (p< 0.05)
Table 3: Sperm characteristics in control and treatment groups with AgNPs
| Groups | Live range | Dead range | Normal range | Abnormal range |
| Control males |
87.00 ± A 3.48 |
13.00 ± 3.48 C |
90.85 ± 4.04 A |
9.15 ± 4.04 C |
| Males treated for 35 days |
41.75 ±3.71 B |
57.25 ±4.44 B |
26.20 ±1.82 B |
73.80 ±1.82 B |
Note: All results were presented as mean ± standard error. Means showing different letters vertically indicates statistical differences (p< 0.05)
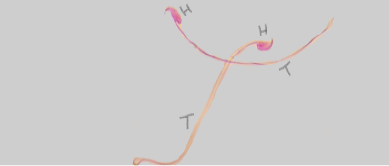
H: Head; T: Tail
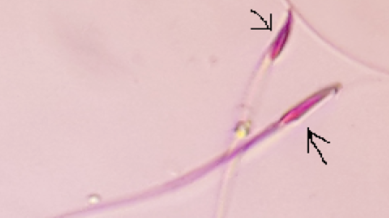
The arrows refers to amorphous head
an increase in the weight of spleen from treated mice (A 0.007± 0.154) than the control (B 0.005 ± 0.132) (Table 1).
Effects of AgNPs on Serum Levels of Testosterone
We found a decrease in serum levels of testosterone in treated group (0.53 ± 1.358) than control (1.93 ± 9.485).
Sperms Characteristic in Control and Treatment Animals
We found a variation in live and dead sperm range in both group of mice. For live sperm, it ranged for treated mice (3.71± 41.75) to control (3.48±87.00). For dead sperm, it varied from (4.44±57.25) for treated group to (3.48±13.00) for control. Further, results revealed that the normal range of sperms in treatment animals was lower (1.82±26.20) than those values in control animals (4.04±90.85). Added to this, abnormal range of sperms in treatment animals was higher (1.82±73.80) than observed in control group (4.04±9.15) (Table 2 and 3).
Histopathological Examination
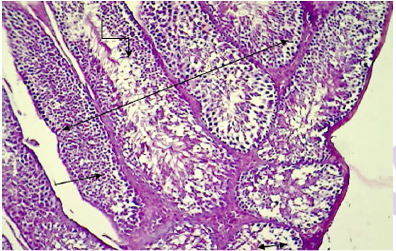
Histopathology revealed normal arrangement of the seminiferous tubules and its epithelium, interstitial connective tissues, spermatozoa in the lumen of the tubules (Figure 1), leydig cells, sertoli cells, myoid cells,spermatogona A and spermatogonia B and spermatocyte (Figure 2, 3 and 4) in the testis of normal mice. Contrary to this, the treated
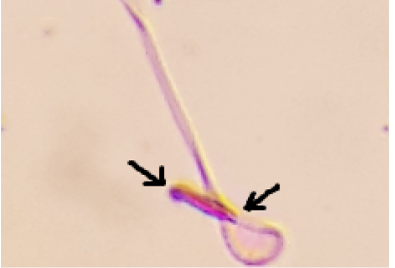
The arrow refers to amorphous with inverted head
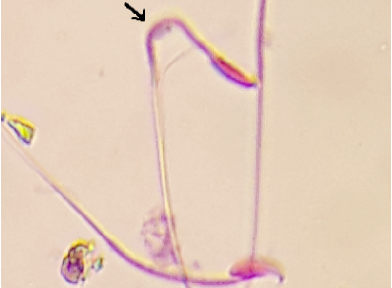
The arrows refers to curved tail
Double headed arrow: Normal arrangement of the seminiferous tubules; Straight line: seminiferous epithelium; Single sided arrow: interstitial connective tissue; Turned line: spermatozoa in the lumen of the tubules (H&E stain 100X).

Figure 6: Histopathological section in testis of control animal shows normal arrangement of the seminiferous epithelium.
mice showed shrinkage and irregular arrangement of seminiferous tubules, increased intertubular space, complete depletion of spermatogenesis,cellular debris, fluid-filled lumen of seminiferous tubules, unilateral deformity of seminiferous tubules with complete spermatogenesis process in one part while incompletespermatogenesis in the other part of testis (Figure 7, 8 and 9).
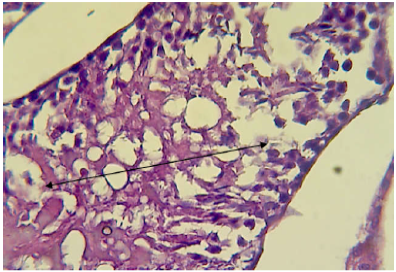
Figure 7: Histopathological section in testis of male animal treated with Ag NPs for thirty five days
It shows completely depleted of spermatogenesis and only cellular debris and fluid filled the lumen of seminiferous tubules sas depicted by doublw head arrow (H&E stain 400X).
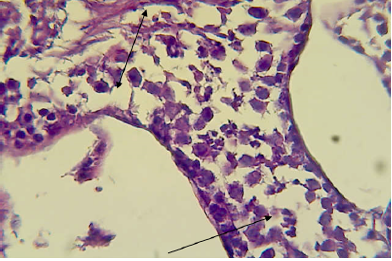
Figure 8: Histopathological section in testis of male animal treated with Ag NPs for thirty five days
It shows deformity of seminiferous tubules i.e one part is wide with complete spermatogenesis process (shown by double headed arrow) and other part is narrow with incomplete spermatogenesis (shown by single headed arrow) (H&E stain 400X).
Discussion
The decrease in body weight indicates the potential toxic effects of AgNPs. The impaired function of internal organs may be associated with the release of proinflammatory cytokines from phagocytic cells upon exposure to AgNPs. Cytokines particularly TNF alpha that are considered associated with increased metabolism of subcutaneous fatty tissue leads to emaciation of the animals. Though Mansee et al. (2014) recorded lack of any sign of toxicity and significant differences in weight of treated and control rats upon exposure to 20g\kg BW of AgNPs for 90 days. The different between our result and the result of Mansee et al. (2014) may be due to the differences in physiochemical properties of nanoparticles used in our experiment. This is in agreement with the observations of Hubbs et al. (2011) who explained that toxicity of nanoparticles depends upon their size and their surface to mass ratio.
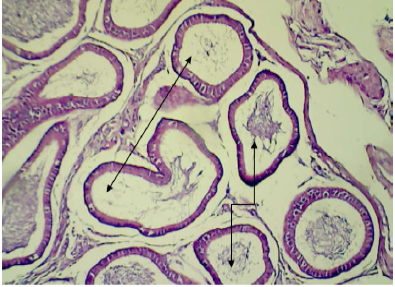
Figure 9: Histopathological section in epididymis of male animal treated with Ag NPs for thirty five days
It shows empty most of their tubules (shown by double headed arrow) and other containing cellular debris and abnormal sperm (shown by turned arrow) (H&E stain 100X).
Here in our study, the decrease in the weight of the testis may correspond to penetration of AgNPs through blood test is barrier and subsequent tissue damage. Similar findings are reported by Hubbs et al. (2011) who explained that the size of nanoparticles play important role in their toxicity. Although some reports suggests that the smaller is the size of nanoparticles, the higher is their toxicity (Chen and Schluesener, 2008), the observation in our study describe otherwise. Our results revealed outcomes similar to what has been reported previously where the surface chemistry of nanoparticles has been identified critical in its interaction and transport the biological membrane (Chithrani and Chan, 2006; Stobiecka et al., 2011).
Increased size in spleen weight may be due to active immune reaction or as a result of inflammatory exudates accumulation. Further a low level of serum testosterone may be due to the potential damage of leydigs cells by AgNPs leading to decreased synthesis and secretion of testosterone. Yoshida et al. (1999) reported that nanoparticles cause damage of leydigs cells and diminish daily production of sperms. Similar observation are reported by Tsukue et al. (2004) and Komatsu et al. (2008). Added to this, Mansee et al. (2014) suggested that nanoparticles may directly interfere with the process of spermatogenesis and can induce genotoxicity.
Allow sperm count together with high percentage of sperm deformity may indicate that AgNPs can destroy the sertoli cells that play an essential role in spermatogenesis process (Boeklheide et al., 2000).
Our results was suggested that 50 nm AgNPs may cause damage to sertoli cells. This correspond to observations made earlier by Borm et al. (2004) and Mansee et al. (2014) who reported accumulation of nanoparticles inside sertoli cells in rats.
Changes in sperm characteristic may be considered as an indicator of response of testis to chemical toxic. Boeklheide et al. (2000) demonstrated changes in the morphology of testis and degenerative changes in sertoli cells as the main signs of response of testis to xenobiotics. Kim et al. (1999) postulated that number of sperms may be considered a good indicator of testicular and spermatogenic damages.
However, not all NP can cause toxic effects on spermatogenesis, certain types of these particles have positive effects on spermatogenesis also (Shi et al., 2010). Shi et al. (2011), reported that goat fed diet supplemented with nanoselenium express improvement in their sperm quality.
The current study revealed loss of normal arrangement of somniferous tubules, with increased in gap between tubules. Added to this, sloughing, necrosis of epithelial cells of somniferous tubules, loss of complete spermatogenesis process, filling of tubules with fluid and cellular debris and decrees in number and size or disappear of leydigs cells. Similar findings has been reported by Dhermendra et al. (2011). Upon administration of nanoparticles for 90 days, Mansee et al. (2014) recorded atrophic changes, sloughing, decreased in germ cells of somniferous tubules and spermatozoa in testis of male rats. We found mononuclear cells present in the lumen of somniferous tubules that may be due to condensation of degenerative spermatozoa. Holstein et al. (1986) recorded multi-nucleated giant cells in the lumen of somniferous tubules of rats exposure to Zink nanoparticles. They suggested that the giant cells result from clumped spermatogenic cells which lost their contact to sertoli cells. The Presence of fluid in the lumen of somniferous tubules and damage to the efferent duct of the testis in our study may have resulted in in retention of the lymphatic fluid along with the deformity of seminiferous tubules largely due to toxic effects of AgNPs on cell membrane. This is in agreement to Xiao et al. (2013) and Gozde et al. (2012) who found that nanoparticles can induce injury or disrupt the functional basement membrane responsible for maintenance of the integrity of testis tissues.
The absence or low sperm counts in the epididymal tubules coincidence with pathological lesions in the test is characterized by a few or absent of spermatogonia, primary and secondary spermatocytes and spermatozoa. This result may indicate that the AgNPs inhibit spermatogenesis or destroyed the germ cells and sertoli cells (Mesbah et al., 2008; Gozde et al., 2012), investigated that nanoparticles can induce damage to basement membrane, sertoli and spermatogonial cells and subsequently leading to deformed spermatocytes and spermatozoa as well as decrease in number of these cells.
The current study revealed that administration of mice with 10mg\kg BW of AgNPs cause severe damage of the testis. Tiwari et al. (2011), described that toxic effects of nanoparticles are dose dependent. They found that more than 20mg\kg nanoparticles are more toxic than 10mg\kg that are safe for biomedical usage. Lee et al. (2013) reported that mice with dose of 30mg\kg nanoparticles do no express acute toxicity but those given 120mg\kg. With the comments that size, duration of exposure, type of nanoparticles and route of administration play an essential role in nanoparticle toxicity, they reported inflammatory reaction in the lung and liver. and they, Finding of the current study that 50 nm size of AgNPs express high toxicity on testis tissue, decrease levels of serum testosterone, induce abnormality and death of the sperms reveals that size of nanoparticles alone do not play role in their toxicity. Similar observations are reported by Chithrani et al. (2006) who described that increasing toxicity of nanoparticles does not dependent on their small size but also surface chemistry of nanoparticles, their distribution (Stobiecka et al., 2011) and genotoxicity (Ahamed et al., 2008).
Conflict of Interest
There is no conflict of interest.
Authors contribution
All authors contributed equally.
References