Journal of Animal Health and Production
Research Article
Fertility Response and Progesterone Profile in Artificially Inseminated Crossbred Ewes following Intravaginal Application of Misoprostol and Isosorbide Mononitrate-A Preliminary Study
Fabiha Rasool1, Farooz Ahmad Lone1*, Mohamad Naiem Banday1, Muzamil Rashid1, Asloob Ahmad Malik1, Hakim Athar2
1Division of Animal Reproduction, Gynaecology & Obstetrics, Faculty of Veterinary Sciences and Animal Husbandry, SKUAST-K, Shalimar, Srinagar, Jammu & Kashmir, India, 190006; 2Division of Veterinary Surgery and Radiology, Faculty of Veterinary Sciences and Animal Husbandry, SKUAST-K, Shalimar, Srinagar, Jammu & Kashmir, India, 190006.
Abstract | In this study, we tested the hypothesis that use of misoprostol and isosorbide mononitrate would improve cervical penetrability and fertility after artificial insemination (AI) with chilled semen. Twenty four crossbred ewes were randomly assigned to three groups; CON (control), MIS (misoprostol) and IMN (isosorbide mononitrate). Estrus was induced by placing progesterone sponges in vagina for 11 days along with an injection of PGF2α 24 hours before sponge removal. Cotton sponges loaded with glycerol, misoprostol and isosorbide mononitrate were placed intravaginally in respective groups at 36 hours and then fixed time AI was done twice at 48 and 60 hours after progesterone (P4) sponge removal using 12-24 hour old chilled semen. Blood samples were collected on the day of sponge insertion, day 0, 15 and 35 for P4 measurement. The pregnancy and lambing rates tended to be higher (p>0.05) in the IMN group (62.5%) compared to the MIS and CON groups (25% each).Interestingly, low P4 concentration (p<0.05) was observed in pregnant ewes on days 15 and 35 than day of sponge insertion possibly indicating lower steroidogenic capacity of early pregnancy corpus luteum compared to cyclic corpus luteum. In conclusion, use of NO donor (IMN) intravaginally 12 hours before AI improved fertility.
Keywords | Cervical ripening agents, Estrous synchronization, Ewes, Fertility, Isosorbide mononitrate
Editor | Asghar Ali Kamboh, Sindh Agriculture University, Tandojam, Pakistan.
Received | January 03, 2019; Accepted | February 03, 2019; Published | February 25, 2019
*Correspondence | Farooz Ahmad Lone, Division of Animal Reproduction, Gynaecology & Obstetrics, Faculty of Veterinary Sciences and Animal Husbandry, SKUAST-K, Shalimar, Srinagar, Jammu & Kashmir, India, 190006; Email: dr.farooz462@gmail.com
Citation | Rasool F, Lone FA, Banday MN, Rashid M, Malik AA, Athar H (2019). Fertility response and progesterone profile in artificially inseminated crossbred ewes following intravaginal application of misoprostol and isosorbide mononitrate-a preliminary study. J. Anim. Health Prod. 7(1): 25-31.
DOI | http://dx.doi.org/10.17582/journal.jahp/2019/7.1.25.31
ISSN | 2308-2801
Copyright © 2019 Rasool et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Effective artificial insemination (AI) in sheep can extend the use of superior rams and reduces the risk of genital infections like vaginitis, cervicitis and endometritis which are common during natural mating (Najafi et al., 2014; Leethongdee et al., 2007). The development of artificial insemination and the subsequent genetic improvement of farm animals have led to a remarkable increase in the productivity of livestock (Najafi et al., 2014), but unfortunately AI in sheep has not been very successful (Salamon and Maxwell, 2000; Anel et al., 2005). For a long time, the standard procedure of inseminating ewes has been to deposit fresh or chilled semen in the external os of the cervical canal; however, suboptimal pregnancy rate is still a major challenge. Although laparoscopic intrauterine insemination has been proposed as an alternative method for AI using frozen-thawed semen with acceptable pregnancy rate (Anel et al., 2005) but its cost, technical expertise and animal welfare have limited its use under field conditions.
The ovine cervix is a highly complex fibrous tubular structure with convoluted lumen (Kumar and Naqvi, 2014) having 2-7 rings (Kershaw et al., 2005), which normally act as barriers to protect the uterus from external contaminants. However, these cervical rings offer the greatest obstacle to the transcervical AI (TCAI). The first, second and third cervical ring are not aligned properly with second ring most misaligned (Naqvi et al., 2005; Kaabi et al., 2006; Aral et al., 2011). With the result the insemination pipette gets misdirected from the central lumen and cannot penetrate further, limiting the deposition of chilled or frozen semen into uterine body. Further, the depth from the os to the first cervical fold has also been implicated as a cause of unsuccessful cervical penetration (Eppleston and Maxwell, 1993; Eppleston et al., 1994).
The Standardized Guelph system for TCAI developed by Buckrell et al. (1994) has several limitations. Several studies (see reviews of Robinson et al., 2011 and Candappa and Bartlewski, 2011) have reported a significant risk of cervical puncture, increasing the chance of infection and greatly decreasing fertility associated with this technique. Therefore, cervical ripening with chemicals either to allow TCAI or to affect fertility offers a plausible strategy to enhance pregnancy rate with AI in sheep. Cervical ripening involves enzymatic breakdown of the connective tissues and relaxation of its smooth muscle fibers. Thus, using chemical substances to promote cervical ripening and dilatation may ease the penetration of the cervix during TCAI. Extensive research has been documented on the use of substances for inducing cervical dilatation in ewes. These substances include oxytocin (Khalifa et al., 1992), human interleukin- 8 (Croy et al., 1999), isosorbide mononitrate (Robinson et al., 1999), misoprostol (Leethongdee et al., 2007; Horta et al., 2010; Falchi et al., 2012), dinoprostone (Candappa and Bartlewski, 2014), with varying degrees of success. Their use either to facilitate TCAI or to affect fertility has been extensively studied with conflicting results. Further, effect of nitric oxide donors on fertility has not been studied in sheep.
Misoprostol, a prostaglandin E1 analogue, stimulates remodeling of extracellular collagen with activation of collagenase, increases water content and hyaluronic acid, decreases the dermatan sulfate and changes the glycosaminoglycan of the extracellular matrix (Smith, 2007).All of these changes result in softening, effacement, and marked relaxation of the smooth muscle fibers resulting in dilatation of the cervix (Stephenson and Wing, 2015).
Nitric oxide (NO) donors have been tried as agents for cervical ripening in humans (Chwalisz et al., 1994, 1997; Facchinetti et al., 2000). The exact mechanism by which NO donors induce cervical ripening is still obscure. However, there is an ample evidence that NO donors cause induction of COX-II leading to synthesis of PGE2 which causes increased vasodilation and thus relaxes the cervix (Chwalisz and Garfield, 1998). Isosorbide mononitrate, a NO donor, has been used for cervical ripening in humans (Pallavi et al., 2013; Agarwal et al., 2014; Dave et al., 2015) and sheep (Robinson et al., 1999) respectively for reducing the time of labor and to promote cervical dilatation for transcervical embryo recovery.
The importance of estrous synchronization together with AI is increasing; however, results from different attempted protocols have not been consistent with respect to fertility. Further, the results obtained with the use of different cervical ripening agents either to facilitate transcervical AI or to enhance fertility after cervical AI have been discouraging. Also isosorbide mononitrate has never been tried as an agent either to facilitate TCAI or to affect fertility in sheep, therefore, we formulated a hypothesis that use of misoprostol and Isosorbide mononitrate would improve cervical penetrability and fertility after artificial insemination with chilled semen in cross-bred ewes.
MATERIALS AND METHODS
Study Location, Selection and Treatment of Animals
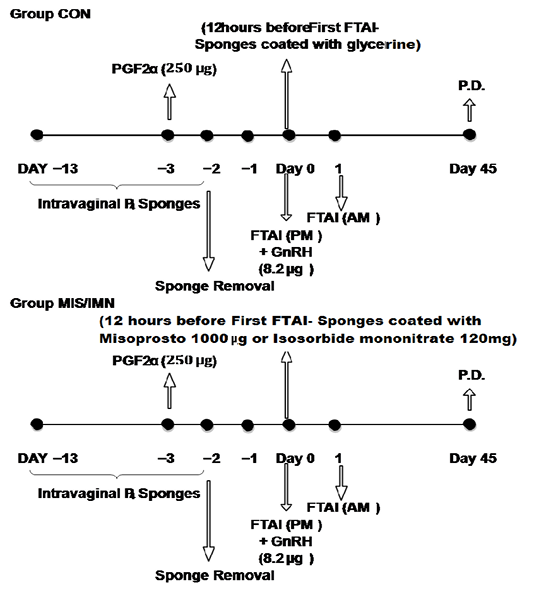
The study was conducted after the onset of breeding season which corresponds to autumn (September to November) in the temperate climate at Mountain Research Station for Sheep and Goat, FVSc & A.H, Shuhama. A total of 24 healthy crossbred ewes (1-3 years of age) with body condition score of 3 and mean body weight of 34.50 ± 6.00 kg (NARI-Swarna ram × non-descript ewe) were selected randomly and distributed in three groups designated as CON, IMN and MIS. Out of 24 ewes, six were one year of age and were distributed equally among the three groups. Ewes in all the three groups received a common estrus induction protocol. Progesterone impregnated Intravaginal sponges (CSWRI, Avikanagar) were placed in the vagina of each ewe with the help of speculum and introducer. An intramuscular injection of PGF2 α (250 µg) was given to all ewes at 24 hours before the sponge removal. The diagrammatic representation of treatment protocols is presented in Figure 1. The experimental animals were grazed on green pastures from 9:00 am to 3:00 pm till November 15 and then onwards hay (oats + sorghum) @ 1.5 kg and concentrate feed (Agrofeed Industries J&K Ltd.) @ 300g were provided to each ewe per day till January. From January the concentrate feed was increased to 600g/day/pregnant ewe. All the ewes had free access to water and salt licks. The ewes were not allowed to come in contact with the rams during the entire study. All animals were ethically handled throughout the study.
Preparation and Insertion of Intravaginal Drug Delivery Cotton Sponges
Intravaginal drug delivery sponges were made using cotton wrapped with cotton gauge and tied to a thick string. Each ewe in the MIS group received 1000µg of misoprostol and the ewes in group IMN received 120 mg of isosorbide mononitrate. For this, 4 tablets (30 mg each) of isosorbide mononitrate (Monit-SR-30) and 5 tablets (200 µg) of misoprostol (Cytotec) were crushed separately using a pestle and mortar. The drugs were mixed individually with 1 ml of glycerin to form a paste as per the method described by Horta et al. (2010). This paste was then applied over the intravaginal drug delivery sponges and left to dry in shade for 10-15 minutes. Some sponges were coated with glycerine only for control group. The other sponges were inserted into the vagina of ewes of respective groups using the speculum and introducer. The sponges were placed 12 hours before and removed 2 hours before first FTAI was performed, thus retained in the vagina for 10 hours only.
Collection of Semen
Three healthy breeding crossbred rams (NARI-Swarna males × non-descript females) were used for collection of semen. Three semen samples were collected early in the morning by artificial vagina method and mixed with tris extender in the ratio of 1:7 and were evaluated for sperm motility. All the three ejaculates were having motility >70% and were thus pooled. The concentrationof the pooled sample was determined and adjusted at 300 million per ml. The samples were stored at 4oC for 12-24 hours before artificial insemination was performed.
Artificial Insemination
All the ewes were subjected to first FTAI 48 hours after progesterone sponge removal or 2 hours after removal of drug delivery sponges in treatment and control groups. For this, 0.5 ml of the semen sample containing approximately 150 million sperm was pipetted using a self-made insemination pipette. The insemination pipette was made by attaching a disposable AI sheath to a 5 ml disposable syringe. A mark was made on the sheath for 0.5ml volume. The hindquarters were lifted and the vagina was dilated using a vaginal speculum to locate the cervical os. Then, the pipette containing semen was introduced into the vagina and semen was deposited at external os of cervix. Insemination was repeated 12 hours later.
Evaluation of Cervical Penetrability
At the time of AI, the ease of penetration of AI gun was evaluated and graded as:
Partial – when the gun penetrated up to two cervical folds.
Middle – when the gun penetrated up to the middle of the cervix.
Complete – when the gun penetrated completely
Pregnancy Diagnosis
On Day 45, all the ewes were examined for pregnancy with real time B mode ultrasonography using 3.5 MHz transducer.
Blood Sampling For Serum Progesterone Estimation
Ten ml of blood was collected from ewes of all the groups in 15 ml centrifuge tubes without anticoagulant. The tubes were kept in slanting position for 1-2 hours. Serum was harvested by centrifugation at 4000 rpm for 15 minutes. Serum was removed from tubes with the help of micro- pipette and stored in 2ml micro-centrifuge tubes at -20oC till further analysis. The blood was collected at the time of insertion of intravaginal P4 sponges (day of first treatment), Day 0 (day of AI), Day 15 and Day 35.
Recording Of Fertility Parameters
The fertility of ewes was determined on the basis of following parameters-
Pregnancy rate=
Number of ewes found pregnant on day 45
Total number of ewes inseminated
Lambing rate=
Number of ewes undergone successful lambing
Total number of ewes inseminated
Prolificacy =
Total number of lambs born
Total number of lambed ewes
Estimation of Serum Progesterone
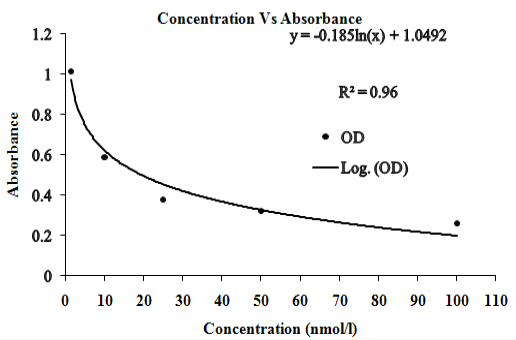
P4 was estimated in the serum samples using “DS-EIA-STEROID-PROGESTERONE” kit, Italy. The “DS-EIA-STEROID-PROGESTERONE” kit method is a one-step immunoassay to determine the amount of P4 in the serum using competitive microplate enzyme immunoassay. Concentration of P4 in unknown serum samples was determined from the standard curve (Figure 2).
Statistical Analysis
The data obtained in respect of time to onset of estrus, estrus response rates, pregnancy rates, lambing rates and prolificacy rates was analyzed by Chi-square test. Mean serum progesterone concentration at different stages within non pregnant and pregnant ewes was pooled irrespective of the group and was analyzed by One-way ANOVA. Post-hoc analysis was done by Duncan multiple range Test. Variation in means between pregnant and non-pregnant groups at different stages was analyzed by two tailed paired T-test. P-value ≤ 0.05 was considered significant (SPSS, Statistics-21).
RESULTS
Cervical Penetrability
Cervical penetrability was determined in all the three groups based on ease of penetration of AI gun. There was no significant difference in the cervical penetrability among treatment groups and control. In all the three groups of animals, the AI pipette could traverse only upto the first cervical fold, thus was graded as partial.
Pregnancy, Lambing And Prolificacy Rate
The overall pregnancy rate, lambing rate and prolificacy of artificially inseminated crossbred ewes are shown in Table 1. The overall pregnancy and lambing rates were non-significantly (P>0.05) higher in the IMN group (62.5%, 62.5%) than CON and MIS groups (25%, 25% each). The prolificacy was non-significantly (P>0.05) higher in group MIS (150%) than group IMN (120%) and group CON (100%).
Progesterone (P4)
The values of mean serum P4 concentration (ng/ml) in pregnant and non-pregnant crossbred ewes of all the three groups were pooled and are presented in Table 2. The mean serum P4 concentration was significantly higher only at day 35 in pregnant compared to non-pregnant ewes. Further in pregnant ewes there was a significant decrease (P<0.05) in serum P4 concentration from Day of P4 sponge treatment to Day 0 and a significant increase (P<0.05) from Day 0 to Day 15 which further increased (P>0.05) upto day 35. Interestingly, mean serum progesterone concentrationat Days 15 and 35 in pregnant ewes was significantly (P<0.05) lower than at Day of P4 sponge treatment.
Table 1: Fertility (Pregnancy, lambing and Prolificacy rate) of artificially inseminated cross bred ewes after using different cervical ripening agents
| Treatment groups | Fertility parameters | ||
| Pregnancy rate (%) | Lambing rate (%) | Prolificacy rate (%) | |
| Group CON (N=8) | 25 (2/8) | 25 (2/8) | 100 (2/2) |
| Group MIS (N=8) | 25 (2/8) | 25 (2/8) | 150 (3/2) |
| Group IMN (N=8) | 62.5 (5/8) | 62.5 (5/8) |
120 (6/5) |
Table 2: Serum progesterone profile (mean ±SEM) in non-pregnant and pregnant crossbred ewes at different stages of AI irrespective of cervical ripening agent used
|
Stage
|
Progesterone Concentration (ng/ml) | |
| Non-Pregnant (N=14) | Pregnant (N=9) | |
| Day of P4 sponge treatment |
18.73 ± 2.08a |
18.84 ± 2.93a |
| Day 0 (Day of first AI) |
0.45 ± 0.09b |
0.42 ±0.05b |
| Day 15 |
2.88 ± 0.52b |
5.41 ± 0.95c |
| Day 35 |
1.95 ± 0.53bA |
7.53 ± 1.35cB |
Means with different superscripts (a, b, c, d) within columns and (A, B) within rows differ significantly (p< 0.05)
DISCUSSION
Success of artificial insemination in ewes has been limited by the anatomy of the sheep cervix as well as poor post thaw semen quality of the ram semen. This study was conducted to gain insight of how cervical ripening agents affect fertility. Although cervical penetrability was not affected, the use of NO donor did increase the pregnancy rate possibly through alternate mechanisms. This study, although preliminary provides an opportunity to further explore the role of NO donors in enhancing fertility following FTAI in sheep.
In the current study we observed that the pregnancy and lambing rate in the IMN group although non-significant was quite higher and promising. In vitro studies have provided substantial evidence that entry of NO into the spermatozoa causes increased intracellular cGMP and hence protein kinase-G leading to hypermotility of the sperm and thus increased chances to reach the site of fertilization (Miraglia et al., 2011; Maidin et al., 2014). Thus passage of the hypermotile sperm through the channels created by copious cervical mucus might be responsible for higher fertility. This is further supported by the experiments carried in different species by Herrero et al. (1994) on mouse sperm, Hassanpour et al. (2007) on ram sperm, Hellstrom et al. (1994) on human sperm. Hyperactivation of mouse sperm has been achieved by Herrero et al. (1994) following supplementation of exogenous NO into the capacitating medium. In similar experiments conducted on ram semen and human semen by Hassanpour et al. (2007) and Hellstrom et al. (1994) respectively, higher sperm motility was recorded in presence of sodium nitroprusside (nitric oxide donor) at low concentration. Similar results have been obtained for buck semen by Maidin et al. (2014) following supplementation of semen with L-arginine, the substrate for nitric oxide production. Here we propose the reasons that might have affected the fertility outcome in IMN group: a) presence of copious cervical mucus at the time of AI, produced by cervical glands under the vasodilatory effect of NO constantly liberated from isosorbide mononitrate over a period of 12 hours might have created a channel for increased sperm transport via the narrow tortuous cervix to reach the site of fertilization. b) The presence of copious mucus in the vagina might have changed pH from the harsh low to suitable high, thus increased survival of sperm in the vagina. c) Nitric oxide released from isosorbide mononitrate might have entered into the spermatozoa resulting in hypermotility.
Since this is the first report to be documented for the use of isosorbide mononitrate for AI in sheep and might open new avenues where researchers can shift their strategy of finding an alternative to TCAI in the form of an agent that can simply enhance the increased transport of spermatozoa across the ewe cervix. Such a strategy would not require any expertise for carrying out TCAI and can be rather easily practiced by a sheep breeder.
The ease of penetration was found to be partial in all the groups, with no significant difference between the two treatment groups and control. Other authors also did not obtain any significant effect on cervical penetration in ewes after local administration of FSH, misoprostol or oxytocin (Falchi et al., 2012), following treatment with misoprostol plus terbutaline (Horta et al., 2010) or after the use of isosorbide mononitrate for embryo recovery (Robinson et al., 1999). However, the use of misoprostol plus terbutaline significantly improved fertility which has been attributed to improved motility in smooth muscles of the genitalia by terbutaline sulphate (Horta et al., 2010). Several others, however, obtained significant improvement in cervical dilatation following use of isosorbide mononitrate at term in women (Facchinetti et al., 2000; Ekerhovd et al., 2003; Abdellah et al., 2010; Pallavi et al., 2013). The variation in the results might be attributed to the species difference and thus different nature of the cervix.
The mean serum progesterone concentration was significantly higher in pregnant than non-pregnant ewes at Day 35. The major source of progesterone during early pregnancy in ewes is corpus luteum (Wiltbank et al., 2014), its survival and release of Progesterone is critically required for establishment and maintenance of pregnancy (Lee et al., 2016). The most important finding regarding P4 concentration was significantly lower mean progesterone concentration at Day 15 and 35 in pregnant ewes compared to levels on Day of insertion of progesterone sponges possibly indicate decreased ability of pregnant corpus luteum to synthesize sufficient amount of progesterone. Although the levels increased from day 15 to Day 35 in pregnant ewes but the levels were not higher than the mid cycle CL. Similar finding was also reported by Davies and Beck, (1993) during early pregnancy in sheep when compared to cyclic CL. A remarkable paper published 30 years before by Rodgers et al. (1988) in the Journal of Reproduction and Fertility reported a lower steroidogenic capacity of CL during late pregnancy (140 days) than that of the estrous cycle (mid-luteal phase) owing to reduction in the content of cytochrome P-450 scc and adrenodoxin. The probable reason for less steroidogenic capacity of early pregnant corpus luteum reported in this study might be similar to what Rodgers et al indicated. This may have implications on early embryo survival in sheep (Bridges et al., 2013). Conclusively, use of isosorbide mononitrate, a nitric oxide donor positively impacts fertility in artificially inseminated ewes through mechanisms other than cervical dilatation. Further, the results probably indicate reduced steroidogenic capacity of early pregnant corpus luteum in sheep. Moreover, this study has opened a new window for other researchers to study isosorbide mononitrate for enhancing pregnancy rate with AI in sheep.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
ACKNOWLEDGEMENTS
The authors would like to thank I/c Mountain Research Centre for Sheep & Goat (MRCSG), FVSC & AH, Shuhama for sparing animals for this experiment.
AUTHORS CONTRIBUTION
All authors contributed significantly to this manuscript and agree with the content. The corresponding author conceived the concept and drafted the paper, Fabiha Rasool, Mohamad Naiem Banday and Muzamil Rashid carried out the trial, Hakim Athar performed USG for pregnancy diagnosis, Asloob A. Malik and Mehrajuddin Naikoo assisted in the trial and compiling of results.
REFERENCES